On April 28, 2011, amendments were made to this protocol. To view the amendments, please see the section titled “Summary of Protocol Amendments.”
Background and Objectives for the Systematic Review
Background
The Original Nomination: The original nomination for this review was for a comparative effectiveness review of the risks and benefits for elderly patients taking cardiovascular medication concomitantly with herbal supplements. As preliminary searching indicated scarce data in this subgroup, the topic for this review was broadened to address the benefits and risks of dietary supplement use in adults taking cardiovascular drugs. Given the rising prevalence of both heart disease and complementary and alternative medicine (CAM) use in the United States, which includes dietary supplementation, a request to evaluate the benefits and risks of combining dietary supplements and cardiovascular drugs is highly relevant. A current and comprehensive synthesis of the relevant published literature will describe where there is evidence of a potential change in efficacy or harms with drug-supplement combinations and identify combinations that have evidence of safe coadministration. Such a review will be of value to both health care workers and consumers.
The Magnitude of the Problem: The American Heart Association estimates that more than 81 million American adults (one-third of all American adults) have at least one form of cardiovascular disease.1 Thirty-eight million of these adults are estimated to be over the age of 60. In these patients, medication burden represents a significant challenge with respect to both adherence and potential for serious drug interactions. Patients with hypercholesterolemia and hypertension, for example, take an average of 3.6 ± 3.7 prescription medications.2 Risk for nonadherence and drug interactions inevitably increases with the number of medications taken, and this is of perhaps greatest concern in the elderly.3,4
The use of CAM, which refers to preventive and therapeutic modalities not generally considered to be part of conventional medicine,5 has increased dramatically in North America over the past decades in both the general and cardiovascular disease populations.6-8 A 2007 national health survey from the United States reported an astonishing $34 billion in annual out-of-pocket expenditures were spent on CAM.9 Of this amount, 44 percent of all out-of-pocket costs for CAM was spent on nonvitamin, nonmineral, natural products.9 Recent estimates suggest that approximately one-third of people suffering from heart failure or other cardiovascular disease use some form of CAM.8,10
Dietary supplementation, including supplementation with herbal and traditional remedies, is a form of CAM. Dietary supplements have become popular for treatment and prevention of illness, with estimates suggesting use by over half (53%) of American adults.1 Data from the National Health Interview Survey suggest that approximately 20 percent of the population with cardiovascular disease in the United States used dietary supplements in 2002 and that 10 percent of respondents using CAM (18% of total respondents reporting use of herbal products) were doing so specifically for cardiovascular conditions.8 Dietary supplementation is particularly common for people suffering from congestive heart failure, ischemic heart disease, and atrial fibrillation, among others.11,12 Importantly, in patients with heart disease, concomitant use of dietary supplements and prescription drugs is substantial, with 58 percent of people taking these supplements at risk for potential adverse effects.11
Drug-Dietary Supplement Interactions: Understanding potential for drug-supplement interactions and consequences of such interactions is essential for clinicians and patients when advising or deciding to add supplements to medication regimes. The use of some dietary supplements may complement standard care. For example, coenzyme Q10 appears promising in treating dyslipidemia. Amongst other actions, coenzyme Q10 has been shown to lower cholesterol, specifically low-density lipoprotein cholesterol,7 and may augment the activity of conventional lipid-lowering medication.
Negative consequences of drug-supplement interactions can influence clinical and surrogate outcomes. St. John’s wort, for example, which is frequently used for the treatment of depression, is a potent inducer of the CYP3A4 enzyme.13 This enzyme is involved in the oxidative metabolism of many synthetic drugs,14-20 including cardiac glycosides, β-adrenergic blockers, calcium channel blockers, statins, angiotensin receptor antagonists, and anticoagulants.11,15. Inducing the metabolism of any of these drugs by this popular herbal product could reduce their efficacy and potentially result in serious clinical consequences.
Dietary supplements can also affect surrogate outcomes, such as activity of factors within coagulation pathways. For example, garlic, fish oil, fenugreek, saw palmetto, gingko biloba, and danshen have been shown to inhibit blood coagulation.11,15,20,21 When coupled with common anticoagulants like aspirin, warfarin, or ticlopidine, increased bleeding is a serious potential risk. Conversely, other supplements such as green tea extract, which contains vitamin K, may inhibit the anticoagulant effects of certain drugs and thus limit their effectiveness.14
Understanding the Difference Between Drug-Drug and Drug-Dietary Supplement Interactions: In the clinical setting, drug-supplement interactions are more difficult to interpret than drug-drug interactions for a variety of reasons. The variability in dietary supplement constituents, quality, and dosage makes it difficult to compare and predict the outcome of an interaction based on single clinical studies or case reports, particularly when different products or manufacturers are involved. When these studies or case reports come from different populations of patients, the validity of extrapolation becomes even more questionable. The dose, duration of treatment, and route of administration all play essential roles in predicting the likelihood of a drug-supplement interaction.
Our knowledge of potential drug-supplement interactions comes from both direct and indirect evidence. Direct evidence includes experimental and observational clinical studies regarding positive or negative shifts in clinical outcomes. Indirect evidence includes pharmacokinetic and/or mechanistic studies whereby surrogate measures of metabolic and/or pharmacodynamic changes suggest a likely drug-supplement interaction. While recommendations made from direct evidence are more robust, available evidence in this field comes largely from studies providing indirect evidence. For instance, important sources of information (in addition to controlled clinical trials assessing drug-supplement combinations) include pharmacokinetic studies on healthy volunteers employing probe drugs for metabolizing enzymes to test for shifts in drug metabolism.19,20. Given the clinical relevance of this topic and the possible lack of clinical information, it is essential that any review on the issue of drug-supplement interactions evaluate both direct and indirect evidence.
Existing Reviews Related to This Topic: Recent systematic reviews related to this topic have been published but do not address the same scope, are not comprehensive, or involve different populations of interest. For example, Mills and colleagues published a systematic review in 2005 focused on the effect of natural health products on the metabolism of conventional medicines.20 Their focus was not cardiovascular drugs or patients, and they did not investigate other modalities of drug interaction or their outcomes in patients. Also in 2005, Desai and colleagues (on behalf of the National Institutes of Health) conducted a review of interactions between dietary supplements and antiplatelet agents.22 The most relevant published systematic review by Izzo and colleagues examined clinical reports of interactions between herbal supplements and cardiovascular drugs.14 Their search was limited to MEDLINE from January 1966 to February 2003 and five relevant textbooks. We are aware of more than 20 relevant publications since this review was published. The same group published another systematic review in 2009 limited to interactions between conventional prescribed drugs and seven commonly used herbal supplements (garlic, ginko biloba, ginseng, kava, St. John’s wort, echinacea, and saw palmetto).6 The most recent relevant systematic review published in 2010 by Kennedy and Seely examined herb-drug interactions identified in clinical trials and the herbal impact on hepatic metabolism via cytochrome P450 isoenzymes.19 While comprehensive, the target population was not specific to cardiovascular patients or drugs, and the review evaluated indirect evidence limited to herbs metabolized via the P450 system.
While some of these existing reviews address relevant components of the current topic, a current, comprehensive systematic review specific to patients with cardiovascular disease is lacking. This synthesis will evaluate literature that addresses meaningful clinical outcomes, as well as important surrogate or intermediate outcomes, in the context of patients taking both cardiovascular drugs and dietary supplements.
Clinical Importance of the Proposed Review: Given the prevalence of cardiovascular disease in the United States and use of dietary supplements in this population, the risk for drug-supplement interactions among patients with cardiovascular disease is substantial. This risk poses a real concern for health care practitioners and patients alike. Due to U.S. drug regulatory policy, much more research and data are available describing drug-drug interactions than drug-supplement interactions, and there is a clear need to synthesize information on drug-supplement interactions.
When considering the use of alternative therapies, patients exercise their right to play an active role in their health care. Similarly, physicians who can offer advice regarding complementary therapies in addition to conventional medicine provide more choice to patients wishing to become more engaged in their health-related decisionmaking. However, both physicians and patients must be able to make informed decisions about combining dietary supplements with cardiovascular drugs. Unfortunately, the information currently available is not always easily accessible in common medical journals or in the tertiary literature. Studies providing direct evidence of interactions between dietary supplements and cardiovascular drugs are small in size and difficult to interpret.6,19 Much of the indirect evidence comes from pharmacokinetic and preclinical studies.
A comprehensive review of literature providing both direct and indirect evidence of drug-supplement interaction in the setting of adults taking cardiovascular drugs will be an important resource for health care practitioners caring for their patients. In turn, we hope that this synthesis will result in health care providers and consumers making more informed decisions when combining dietary supplements with cardiovascular medications to provide benefits without harm.
Objectives
The objectives of the current review are to examine the benefits and harms of concomitant use of dietary supplements in adults taking commonly used cardiovascular drugs (for definitions, see Section IV).
The Key Questions
Introduction
The proposed key questions were posted for public comment on the Agency for Healthcare Research and Quality (AHRQ) Effective Health Care Program Web site from August 16 through September 13, 2010. Briefly, five general comments were as follows: 1) The review scope should be defined to the most common dietary supplements and cardiovascular drugs. 2) The review should focus on patient-oriented outcomes. 3) The review should further examine issues related to quality, dose, and purity of the dietary supplements of interest. 4) The review should distinguish between regular and occasional users of dietary supplements. 5) The review should distinguish between nutrient and non-nutrient supplements. Our general responses to these comments are as follows:
- While the scope is broad, the topic-refinement process suggested that a comprehensive review is lacking and needed. As such, the scope has not been narrowed at this time. The exception is key question (KQ) 5, which focuses on a subset of the most commonly used dietary supplements.
- The emphasis is proposed to be on patient-oriented outcomes. Secondary outcomes include intermediate/surrogate, pharmacokinetic, and pharmacodynamic outcomes.
- The review team strongly agrees that the formulation, quality, purity, and dose of a dietary supplement are integral components to understanding the effects that may be observed in the studies included in the review. Relevant data, where reported, will be extracted to help interpret the internal validity and applicability of a given study. However, it was agreed that planning to comprehensively compare the quality of different marketed products as a primary or secondary objective of this review is beyond its scope.
- The team agrees that distinguishing between different dose regimens and durations of treatment is important; this will be accomplished via subgroup or sensitivity analysis and applicability assessment, where possible.
- All dietary supplements will be treated individually in this review, where possible and appropriate, and results will be organized by using a categorization such as that used in the Dietary Supplement Health and Education Act of 1994 to define dietary supplements (please see below). Thus, we will be distinguishing between different types of supplements in our report.
The remaining seven comments were question-specific; five (one for each key question) shared with us the respondents’ knowledge on the topic and included no suggestions for changes. The remaining requests were as follows: 1) for KQ 1, clarification of how the review will differentiate between varying effects of drug-supplement combinations, as most cardiac patients take multiple drugs of many varieties; 2) for KQ 3, consideration issues related to dietary supplement quality and purity (as above); and 3) for KQ 3, clarification of how harms outcomes will be measured. These points were not deemed to propose changes to the KQs. Points 1 and 2 highlight potential confounding in the included studies, and we acknowledge these concerns. Where substantially complex, multiple, concomitant medications/dietary supplements make inference based on the study results impossible, studies may be excluded altogether or in sensitivity analyses. In response to the third specific comment, harms will not be measured but rather extracted as measured in the included studies.
Two minor changes to the phrasing of the KQs were made to improve clarify, without modifying the essence of the questions.
Finally, the isoenzyme activity profile of uridine diphosphate glucuronosyl transferase has been added as an outcome for KQ 5.
Key Questions
In adults taking cardiovascular drugs, what are the effects of concomitant use of specific dietary supplements (when compared to cardiovascular drugs alone or cardiovascular drugs and a different dietary supplement[s]) on:
Question 1
. . . clinical cardiovascular effectiveness/efficacy outcomes (e.g., mortality and specific cardiovascular or cerebrovascular conditions such as myocardial infarction [MI] and stroke)?
- Do the effect estimates of clinical cardiovascular outcomes vary by age, ethnicity, gender, or health status?
- Is there a measurable interaction between cardiovascular drugs and dietary supplements for clinical cardiovascular outcomes?
Question 2
. . . intermediate cardiovascular efficacy outcomes (e.g., lipids, blood pressure, electrocardiographic measurements, serum markers, bleeding, and coagulation times)?
- Do the effect estimates of intermediate cardiovascular outcomes vary by age, ethnicity, gender, or health status?
- Is there a measurable interaction between cardiovascular drugs and dietary supplements for intermediate cardiovascular outcomes?
Question 3
. . . clinical or intermediate harms outcomes (e.g., organ toxicity, serious adverse events, withdrawal due to adverse events)?
- Do the effect estimates of harms outcomes vary by age, ethnicity, gender, or health status?
- Is there a measurable interaction between cardiovascular drugs and dietary supplements for harms outcomes?
Question 4
. . . pharmacokinetic outcomes (e.g., T1/2, area under the concentration curve [AUC]) of cardiovascular drugs of interest?
- Do the effect estimates of pharmacokinetic outcomes vary by age, ethnicity, gender, or health status?
- Is there a measurable interaction between cardiovascular drugs and dietary supplements for pharmacokinetic outcomes?
Question 5
In the general adult population, what is the clinical evidence (i.e., in vivo studies) that the most commonly used dietary supplements:
- . . . cause alterations in the activity of cytochrome P450 or uridine diphosphate glucuronosyl transferase isoenzymes (i.e., induction or inhibition) involved in the metabolism of cardiovascular drugs used commonly in outpatient settings?
- . . .cause alterations in cellular drug-transport mechanisms (e.g., drug-transport proteins)?
Definitions of Population, Intervention, Comparator, Outcomes, Timing and Setting (PICOTS)
Key Questions 1–4
P: Adults taking commonly used cardiovascular drugs, regardless of underlying disease. Prespecified subgroups are listed in Section IVF (data synthesis).
Classes of commonly used cardiovascular drugs to be considered (excluding drugs used exclusively for noncardiovascular conditions) are as follows: β-adrenergic antagonists, calcium channel antagonists, renin-angiotensin-aldosterone system antagonists, vasodilators, α-adrenergic antagonists, diuretics, antiarrhythmic drugs, inotropic agents, antilipemic agents, anticoagulants, and antiplatelet agents.
Specific drugs of interest are limited to those commonly used in outpatient settings in the United States for treatment and prevention of cardiovascular disorders (see Appendix 1).
I: Dietary supplement (please see Table 1 for definition; Appendix 1 includes a list of dietary supplements of specific interest).
C: No dietary supplement (e.g., no add-on dietary supplement, placebo) or another dietary supplement.
O: Outcome categories (please see below for details):
- Clinical outcomes (KQ 1)
- Intermediate outcomes (limited to established efficacy outcomes) (KQ 2)
- Harms (clinical or intermediate) (KQ 3)
- Pharmacokinetic and pharmacodynamic outcomes (KQ 4)
T: No restrictions have been placed on study duration.
S: No restrictions have been placed on study setting.
Key Question 5 (Indirect Evidence)
P: General adult population (>16 years of age)
I: Most commonly used dietary supplements (see Table 1)
C: No dietary supplement or placebo
O: Pharmacokinetic and pharmacodynamic outcomes
T: No restrictions, as for KQs 1–4
S: No restrictions, as for KQs 1–4
Analytic Framework
Figure 1. Analytic Framework for Dietary Supplement Use in Adults Taking Cardiovascular Drugs.
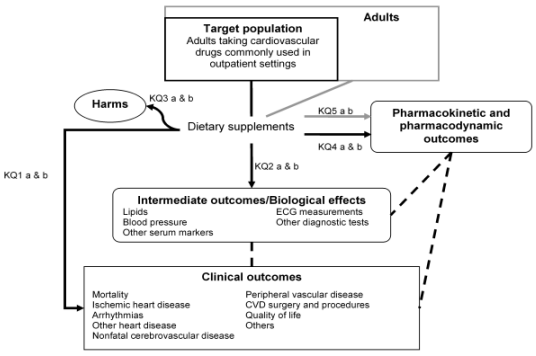
Abbreviations: CVD = cardiovascular disease; ECG = electrocardiography.
Methods
A. Input From Stakeholders
The KQs were developed in consultation with a Key Informant Panel (see Sections VIII and IX) and were posted for public comment. This review protocol was developed in consultation with an external Technical Expert Panel (TEP; (see Section X). A draft report will be prepared by the Evidence-based Practice Center (EPC) and reviewed by a peer reviewer panel (see Section XI). Conflicts of interest will be managed according to standards set by the Agency for Healthcare Research and Quality (AHRQ; see Sections VIII–XI).
B. Criteria for Inclusion/Exclusion of Studies in the Review
This review will focus primarily on hypothesis-testing studies. Broadly defined, eligible studies will be both experimental and observational comparative studies evaluating the benefits or harms of concomitant dietary supplement use in adults taking cardiovascular medications versus no dietary supplement (or other dietary supplement). For a subset of the most commonly used dietary supplements, indirect evidence of dietary supplement pharmacokinetics and pharmacodynamics based on human in vivo studies will also be sought. This subset was developed based on a review of surveys of supplement use in the United States and on nominations by clinical experts (review team and TEP members). This was followed by a two-round modified Delphi consensus survey of TEP members from which the 10 supplements receiving the most votes were selected. This review will be limited to human studies and will exclude studies where either the cardiovascular agent or dietary supplement is added to blood, plasma, or tissue in vitro. Other specific eligibility criteria are presented in Table 1.
A dietary supplement must contain one or any combination of the following substances:
- A vitamin
- A mineral,
- An herb or other botanical
- An amino acid
- A dietary substance for use by man to supplement the diet by increasing the total dietary intake (e.g., enzymes or tissues from organs or glands)
- A concentrate, metabolite, constituent or extract
Furthermore, it must also conform to the following criteria:
- intended for ingestion in pill, capsule, tablet, powder or liquid form not represented for use as a conventional food or as the sole item of a meal or diet
We will include studies only where the supplement(s) of interest is clearly defined and consistent within a study arm or exposure group.
Please see Appendix 1 for a list of preidentified supplements of interest. Others not listed but meeting the definition above will be eligible.
| Characteristic | Key Questions | Criteria |
|---|---|---|
| a Definition is modified from the Dietary Supplement Health and Education Act of 1994;23 the criteria for the product to be “labeled as a dietary supplement” has been omitted for the purposes of this review. b Subset of supplements derived from a review of surveys of supplement use in the United States and nominations by clinical experts (review team and TEP members). See text for details. c The U.S. Food and Drug Administration's (FDA) definition of serious adverse events will be used.24 d The languages of publication eligible for inclusion in this review will be defined in consultation with the AHRQ Task Order Officer after initial screening based on volume of search results and available resources. |
||
| Population | KQs 1–4 | Adult participants (the majority of participants ≥16 years of age or with subgroup data presented for adults) taking at least one specific cardiovascular drug or one specific class of cardiovascular drugs as defined in Appendix 1. The drugs are restricted to those commonly used in U.S. outpatient settings for treatment and prevention of cardiovascular disorders. Where more than one cardiovascular drug is taken by study participants (e.g., where drugs are generally coprescribed), to be eligible for inclusion, all participants must be either uniformly taking concomitant medications, or the study must have controlled for confounding due to concomitant therapy (e.g., distribution of concomitant drugs balanced between study arms, adjusted for in the analysis, or presented by subgroups). Where complex/multiple concomitant medications or dietary supplements substantially confound the study results, studies will be excluded. |
| KQ 5 | Adult participants (the majority ≥16 years of age or with subgroup data presented for adults). | |
| Intervention | KQ 5 | Commonly used dietary supplementsb:
|
| Comparator | KQs 1–4 | No dietary supplement (placebo, no treatment) or another dietary supplement. |
| KQ 5 | No dietary supplement (placebo, no treatment). | |
| Outcomes | KQ 1 | Clinical outcomes:
|
| KQ 2 | Intermediate outcomes (limited to established outcomes):
|
|
| KQ 3 | Harms:
|
|
| KQ 4 and 5 | Pharmacokinetic and pharmacodynamic outcomes:
|
|
| Timing | KQs 1–5 | No restrictions have been prespecified based on the timing of an intervention and/or duration of length of followup of the studies. This will be considered as a dose (or exposure) response modifier in the assessment of heterogeneity and applicability. |
| Setting | KQs 1–5 | No restrictions have been prespecified based on the setting of the studies. This will be considered in the assessment of heterogeneity and applicability. |
| Study Design | KQs 1–4 | High-quality systematic reviews (see below for details) or experimental (randomized or nonrandomized controlled trial) and observational (cohort, case-control, cross-sectional) comparative studies with independent (concurrent or historical) control group including at least five participants. |
| KQ 5 | In addition to the above, uncontrolled before and after studies with at least five participants. | |
| Report Characteristics | KQs 1–5 |
|
Although, based on preliminary scoping of the literature, many studies of relevance are expected to be included in this review, few relevant randomized controlled trials (RCTs) for any one drug-dietary supplement combination are anticipated. Additionally, RCTs may include relatively highly selected populations and may not measure long-term outcomes. Thus, comparative nonrandomized trials and observational studies without major sources of bias may increase the overall applicability of our findings and will be considered for inclusion in this review.
No limits will be placed on publication year or publication status. At this time we will consider reviewing reports of primary studies in languages other than English. After the titles and abstracts of the studies have been screened, the languages of publication eligible for inclusion in this review will be defined in consultation with the AHRQ Task Order Officer (TOO) based on the volume of search results and available resources.
C. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies To Answer the Key Questions
The following electronic databases will be searched: MEDLINE, EMBASE, the Cochrane Library (CENTRAL, Cochrane Database of Systematic Reviews, DARE, HTA), International Bibliographic Information on Dietary Supplements (IBIDS), and Allied and Complimentary Medicine Database (AMED).
Two separate searches will be conducted for this review. The first search will be used to identify studies of relevance to KQs 1–4. Study design and safety filters will be used to identify relevant studies. A supplemental search strategy will be developed for KQ 5.
Electronic search strategies will be developed and tested by an experienced medical information specialist in consultation with the team by using a combination of controlled vocabulary and free text. The sensitivity of the strategies will be tested based on literature identified during scoping exercises for this review and the strategy will be peer reviewed according to the PRESS guideline.25 No language or date restrictions will be imposed on any of the searches. The strategy will be limited to studies in humans or nonindexed in-process citations. The final Cochrane Library search strategy is included in Appendix 2. Strategies will be translated as appropriate for the other databases. The electronic literature search for this review will be updated concurrently with the peer-review process. Methods for incorporating newly identified literature into the report will be discussed with the review TOO.
Grey literature will be identified through a search of trial registries (including ClinicalTrials.gov, Current Controlled Trials, Clinical Study Results, WHO Clinical Trials), the CSA Conference Papers Index, and Scopus. Additional references will be sought by scanning reference lists of relevant systematic reviews, clinical trials, and monographs (e.g., Natural Standard, European Medicines Agency), and by contacting experts.
Citations will be uploaded into DistillerSR,26 a review manager Web application. Reviewers will attend a screening training session, and screening forms will be pilot tested. A broad screen of titles and abstracts will be followed by full-text screening of articles deemed relevant or where eligibility remains unclear. At each level of screening a random sample of at least 10 percent of records will be screened in duplicate independently by a second reviewer. The final proportion of records assessed in duplicate will be dependent on the volume of search output, preliminary assessments of reviewer agreement, and available resources. Discrepancies or ambiguities regarding eligibility will be resolved by discussion or the involvement of another team member, if necessary. The primary reason for exclusion of each record will be recorded.
The process of searching for the evidence for this review will first involve searching for relevant systematic reviews, followed by primary literature. Systematic reviews published in English will be searched by using a slightly modified strategy from that indicated above, including a systematic review filter (for MEDLINE and EMBASE), and by limiting the search of the Cochrane Library to the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, and the Health Technology Assessment Database. We define a systematic review according to the definition used by the Cochrane Collaboration.27
We will assess the relevancy of reviews based on predefined eligibility criteria and will assess the quality of relevant reviews by using the AMSTAR tool,28 as recommended in the AHRQ Methods Guide.29 Reviewers will be trained in the use of the tool and, to be eligible for inclusion, systematic reviews will have to be rated as having adequate quality by two reviewers. Discrepancies will be resolved by discussion with a third reviewer, if necessary. We will perform independent checks on data extraction from a sample of studies included in reviews of adequate quality. Where possible, we will include existing data extraction, risk-of-bias assessments, and syntheses. Where additional data are required to answer the questions of interest, reports of included relevant primary studies will be consulted. In the event that multiple relevant, good-quality reviews are located that address a similar research question, summary data will be presented for each, with focus being given to the most relevant/comprehensive review with the highest overall quality.
D. Data Abstraction and Data Management
Prior to data abstraction, a standardized extraction form will be iteratively developed and pilot tested until an appropriate level of consensus is reached. Data will then be extracted from each study by one reviewer with a second reviewer independently extracting data from a 10 percent random sample (minimum).
Discrepancies will be resolved as described above. Data extractors will not be blinded to study information. Regular meetings will be held to address extraction problems. Further, a sample of data entered into summary tables will be verified against the original report.
If a study is reported in multiple publications, data from the latest and/or most complete publication will be extracted and supplemented by data from companion publications, as appropriate. We will seek additional information from authors when necessary. Data will be extracted by using DistillerSR.26
For all studies, the following data will be extracted, where reported:
- Study and report characteristics: study design, study setting, duration of followup, first author,year and language of publication, funding source.
- Population characteristics: inclusion/exclusion criteria, numbers enrolled and analyzed, participant characteristics (age, gender, ethnicity/race, health status, comorbidities, baseline nutrient exposures and/or background diet), methods used to assess baseline nutrient exposures, specific cardiovascular drug(s) and drug class(es), dose(s), dose regimen(s), duration of treatment, confounders (e.g., blood pressure, concomitant medication, smoking, LDL-C) where not included as study outcomes, and number and reasons for exclusions or drop-outs.
- Intervention characteristics: name (Latin, common, product [e.g., brand, manufacturer], extract, as appropriate), country of manufacture, whether the product used is authorized (licensed, registered) in country of manufacture, characteristics (e.g., source [such as part of plant] used to produce the product or extract, extraction solvent, method of authentication), dosage regimen and quantitative description, dose form, qualitative testing (authenticity of herbal species, purity assessment [contamination/substitution], standardization), storage conditions and length, methods and instruments for assessing nutrient-intake exposures (including validation by using nutrient biomarkers).
- Comparison intervention characteristics: In placebo-controlled trials, description/definition of control, duration of treatment, similarity of treatments. If other dietary supplements are used, same criteria as above apply.
- Outcomes: See Table 1. Data—including baseline value, final value, within-treatment change or between-treatment difference, and variance—will be extracted, as reported. Different combinations of clinical outcomes will likely be reported, and definitions may vary across studies. Outcomes will thus be categorized under common definitions, as judged by a clinician-methodologist member of our research team. In addition to quantitative data for each outcome, definitions, methods used to assess outcomes, and timing of outcome measurement (such as time from randomization) within each study will be extracted.
For categorical variables, we will extract the number of events or number of patients with events among study treatment groups to calculate rates, relative risk, and absolute risk differences (ARD). For continuous outcomes, means and standard deviations will be extracted to calculate mean differences with a 95 percent confidence interval (CI). For RCTs, we will extract, if relevant, the number randomized to each treatment group, the time of assessment from randomization, and the time of followup after initial treatment and after treatment ceased. For observational studies, we will extract crude rates or relative measures of the association (relative risk, hazard ratio, odds ratio) with the standard error or 95 percent CI and reported adjustments for patient age, race, gender, comorbidities, concomitant treatments, or other baseline variables.
E. Assessment of Methodological Quality of Individual Studies
Using generic criteria, risk of bias (quality) will be assessed for each major outcome (Table 2) including selected criteria from the McMaster Quality Assessment Scale of Harms (McHarm)31 for primary studies evaluating harms of treatment. All domains will have response options of “yes,” “no,” or “unclear” and allow justifications of such judgments.
| Bias | Criteria | Experimental controlled trials (RCT and non-RCT) | Observational controlled studies | Uncontrolled experimental (for KQ 5 only) |
|---|---|---|---|---|
| a Appropriateness of participant selection will include whether participants from each arm are from the same or different populations, the use of adequate definitions and measurements of exposed/nonexposed or case/control status and demonstration that outcomes are not present at beginning of prospective studies. b For RCTs only. c Important baseline/prognostic factors to be evaluated: age, gender, race, baseline diet/nutrient exposures for dietary substance of interest, smoking, concomitant medications/supplements, and health status, including comorbidities and LDL-C and blood pressure if these measures are not included as study outcomes. d Description of the source, methods of extraction, and constituents of the dietary supplement (e.g., quantification or quality assurance/standardization of dose/purity, stability and length of storage). e Definition and methods used to assess outcome including timing, and comparability of assessment across study arms. |
||||
| Selection Bias | Appropriateness of participant selectiona | X | X | X |
| Randomization sequence generationb | X | |||
| Allocation concealment | X | |||
| Control for important baseline/prognostic factorsc: restriction, similarity of groups at baseline, matching, and/or adjustment in the analysis | X | X | ||
| Performance Bias | Purity/standardization f dietary supplementd | X | X | X |
| Similarity of overall provider care between study arms | X | X | ||
| Blinding of subjects and providers to treatment allocation |
X |
|||
| Attrition Bias | Completeness of outcome data (attrition and exclusions) with focus on differential loss to followup or overall high loss to followup and potential for associated confoundingc | X | X | X |
| Detection Bias | Blinding of outcome assessors to treatment or exposure status in experimental or cohort studies or blinding of exposure assessors to case/control status in case-control studies. | X | X | X |
| Extent to which valid outcomes were describede | X | X | X | |
| Other | Financial conflict of interest | X | X | X |
| Criteria for assessment of harms (from the McHarm checklist31): prespecified and valid definition of harms, mode of harms collection (e.g., active or passive, standard tool) | X | X | X | |
For major review outcomes (defined below in Grading the Evidence), we will rate the overall risk of bias as low, moderate, or high as defined in Section VI. Selective outcome-reporting bias will be evaluated for experimental and cohort studies and incorporated in the overall assessment of the strength of evidence for important outcomes. For other outcomes, summary assessment of risk of bias will not be made; however, risk of bias (e.g., yes, no, unclear) will be reported for each criterion applicable to the study based on its design. Study quality will be assessed by one reviewer, a second reviewer assessing a random sample; discrepancies will be resolved as described previously.
F. Data Synthesis
Outcomes may be categorized as binary, continuous, count, or time-to-event data. In general, for binary outcomes, risk ratios will be the effect estimate of choice, followed by odds ratios—except in the case of case-control and cross-sectional studies where crude or adjusted odds ratios will be extracted. For continuous outcomes, mean differences or standardized mean differences will be estimated, as appropriate. Count data may be reported and analyzed as rate ratios.
For KQs 1–4, when no important clinical or methodological diversity is deemed to exist between two or more studies for an outcome of interest, we will consider quantitatively synthesizing the data by using a random effects model32 according to guidance described for the AHRQ Effective Health Care Program.33 Statistical heterogeneity will be estimated by computing the Cochran Q and I-squared statistics34 (α = 0.10). When meta-analysis is not deemed appropriate, data will be qualitatively synthesized and heterogeneity highlighted.
When event rates are <1 percent, the Peto odds ratio method will be used. For studies with zero events in one arm, estimation of effect measures will require the addition of a correction factor of 0.5 to all cells except when the Peto method is used. Studies with zero events in both arms will be excluded from meta-analysis.
For KQ 4, where possible, weighted average of the areas under the curve will be meta-analyzed.
KQ 5 will be answered in a qualitative synthesis of the best available evidence.
Exploring heterogeneity, sensitivity and subgroup analyses
Heterogeneity may be explored by using subgroup analyses, sensitivity analyses, or random-effects meta-regression for study-level covariates. Studies of experimental and observational designs will not be meta-analyzed together. Methodological diversity will be explored by adequacy of participant selection, important confounding, blinding of outcome assessors, assessment of the purity/dose/stability of a dietary supplement and, for RCTs, adequacy of allocation concealment. Exploration of clinical diversity may include, briefly, differences in study population, intervention dose, dose form and purity, and outcome definitions and measurement methods. Additional covariates may be examined in a posthoc fashion if required. Exploration of heterogeneity will follow recommendations from the AHRQ Methods Guide.33
Prespecified subgroup analyses are intrinsic to part a of KQs 1–4 and include the following, where reported: gender; ethnicity (including Hispanic, Asian, African American, and Native American); age (those aged ≥65 years and ≥80 years); baseline health status (healthy volunteers; participants at risk for cardiovascular disease, with known cardiovascular disease; participants with diabetes; participants with hepatic or renal dysfunction or end-stage renal disease; participants taking a cardiovascular drug for an indication other than cardiovascular disease); and genotypic polymorphisms (e.g., in CYP2D6, 2C9, 2C19). When possible, subgroup meta-analyses will be presented separately.
Key outcomes for each review question were determined in consultation with the TEP and are included in the following section.
G. Grading the Evidence
The body of evidence for the following important outcomes will be graded as high, moderate, low, or insufficient based on the standard approach for EPCs as outlined in the AHRQ Methods Guide35 (for definitions, please see Section VI):
KQ 1: Mortality (all-cause and vascular death), myocardial ischemic events (fatal MI, nonfatal MI, unspecified MI, and acute coronary syndromes), cerebrovascular events (hemorrhagic/ischemic/unspecified stroke), quality of life, hospitalization, arrhythmia, peripheral vascular disease
KQ 2: Blood pressure (systolic, diastolic), lipid profile (LDL-C, HLD-C, non–HDL-C, triglycerides), international normalised ratio, incidence of metabolic syndrome, change in 10-year Framingham risk profile, and QT prolongation
KQ 3: Serious adverse events (composite outcome—FDA definition of serious adverse events24), withdrawal due to adverse events, clinical bleeding (intracranial, gastrointestinal, genitourinary, subretinal, etc.), renal dysfunction (e.g., proteinuria, raised creatinine, need for transplant, GFR), hepatotoxicity (raised enzymes or fulminant failure)
KQ 4: Area under the concentration curve (AUC), Cmax, clearance (T1/2)
KQ 5: Too indirect to be graded
Two methodologists will rate the strength of the body of evidence across the domains of risk of bias (including risk of selective outcome reporting), consistency, directness, and precision according to published guidance.35 Additional domains may be considered if applicable. Disagreements will be resolved by consensus or adjudication by a third methodologist.
Applicability will be assessed according to the domains of patient population, intervention and dose, comparator, outcome, and study duration and will be reported accordingly.
References
- Writing Group for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 Update: a report from the American Heart Association. Circulation 2010;121:e46-215.
- Benner JS, Chapman RH, Petrilla AA, et al. Association between prescription burden and medication adherence in patients initiating antihypertensive and lipid-lowering therapy. Am J Health Syst Pharm 2010;66:1471-7.
- Maraldi C, Lattanzio F, Onder G, et al. Variability in the prescription of cardiovascular medications in older patients: correlates and potential explanations. Drugs Aging 2009;26(Suppl 1):41-51.
- Garner JB. Problems of nonadherence in cardiology and proposals to improve outcomes. Am J Cardiol 2010;105:1495-501.
- National Center for Complementary and Alternative Medicine. What is complementary and alternative medicine? Available at: http://nccam.nih.gov/health/whatiscam/. Updated November 22, 2010. Accessed December 16, 2010.
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 2009;69:1777-98.
- Miller KL, Liebowitz RS, Newby LK. Complementary and alternative medicine in cardiovascular disease: a review of biologically based approaches. Am Heart J 2004;147:401-11.
- Yeh GY, Davis RB, Phillips RS. Use of complementary therapies in patients with cardiovascular disease. Am J Cardiol 2006;98:673-80.
- Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report 2009 Jul 30;(18):1-14.
- Zick SM, Blume A, Aaronson KD. The prevalence and pattern of complementary and alternative supplement use in individuals with chronic heart failure. J Card Fail 2005;11:586-9.
- Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol 2010;55:515-25.
- Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med 1998;158:2225-34.
- Mannel M. Drug interactions with St John's wort: mechanisms and clinical implications. Drug Saf 2004;27:773-97.
- Izzo AA, Di Carlo G, Borrelli F, et al. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol 2005;98:1-14.
- Coxeter PD, McLachlan AJ, Duke CC, et al. Herb-drug interactions: an evidence based approach. Curr Med Chem 2004;11:1513-25.
- Flattery M. Herbal therapies and cardiac side effects. Prog Cardiovasc Nurs 2008;23:187-90.
- Caccia S, Gobbi M. St. John's wort components and the brain: uptake, concentrations reached and the mechanisms underlying pharmacological effects. Curr Drug Metab 2009;10:1055-65.
- Izzo AA. Herb-drug interactions: an overview of the clinical evidence. Fundam Clin Pharmacol 2005;19:1-16.
- Kennedy DA, Seely D. Clinically based evidence of drug-herb interactions: a systematic review. Expert Opin Drug Saf 2010;9:79-124.
- Mills E, Wu P, Johnston BC, et al. Natural health product-drug interactions: a systematic review of clinical trials. Ther Drug Monit 2005;27:549-57.
- Ramsay NA, Kenny MW, Davies G, et al. Complimentary and alternative medicine use among patients starting warfarin. Br J Haematol 2005;130:777-80.
- Desai D, Hasan A, Wesley R, et al. Effects of dietary supplements on aspirin and other antiplatelet agents: An evidence-based approach. Thromb Res 2005;117:87-101.
- U.S. Food and Drug Administration Web site. Dietary Supplement Health and Education Act of 1994. Available at: http://www.fda.gov/RegulatoryInformation/Legislation
/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148003.htm#sec1. Updated May 20, 2009. Accessed December 16, 2010. - U.S. Food and Drug Administration Web site. What is a Serious Adverse Event? Updated December 15, 2010. Available at: http://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm. Updated December 15, 2010. Accessed December 16, 2010.
- Sampson M, McGowan J, Cogo E, et al. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol 2009;62:944-52.
- Evidence Partners Inc. DistillerSR [Web-based systematic review software]. 2010.
- Green S, Higgins JPT, Alderson P, et al. Introduction. In: Higgins JPT and Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.1. Oxford, England: The Cochrane Collaboration; 2008.
- Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10.
- White CM, Ip S, McPheeters M, et al. Using existing systematic reviews to replace de novo processes in conducting comparative effectiveness reviews. In: Methods Guide for Comparative Effectiveness Reviews. Rockville, MD:Agency for Healthcare Research and Quality; Posted September 2009. Available at: /sites/default/files/pdf/methods-guidance-de-novo-processes_methods.pdf.
- Balk E, Chung M, Lichtenstein A, Effects of Omega-3 Fatty Acids on Cardiovascular Risk Factors and Intermediate Markers of Cardiovascular Disease: Summary, Evidence Report/Technology Assessment No. 93 (Prepared by Tufts–New England Medical Center Evidence-based Practice Center under Contract No. 290-02-0022). Rockville, MD: Agency for Healthcare Research and Quality, March 2004. AHRQ Publication No. 04-E010-1.
- Santaguida P, Raina P, Ismaila A. McMaster Quality Assessment Scale of Harms (McHarm) for primary studies: manual for use of the McHarm. Hamilton, Ontario, Canada: McMaster University. Available at: http://hiru.mcmaster.ca/epc/mcharm.pdf. Accessed December 16, 2010.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol 2011; in press.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
- Owens DK, Lohr KN, Atkins D, et al. Grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol 2010;63:513-23.
- Pharmacodynamics. Wikipedia. Available at: http://en.wikipedia.org/wiki/Pharmacodynamics. Updated December 1, 2010. Accessed December 16, 2010.
- Pharmacokinetics. Wikipedia. Available at: http://en.wikipedia.org/wiki/Pharmacokinetics. Updated October 7, 2010. Accessed December 16, 2010.
Definition of Terms
Pharmacodynamics:
The study of the physiological effects of drugs on the body or on microorganisms or parasites within or on the body and the mechanisms of drug action and the relationship between drug concentration and effect.36
Pharmacokinetics:
The study of the mechanisms of absorption and distribution of an administered drug, the rate at which a drug action begins and the duration of the effect, the chemical changes of the substance in the body (e.g., by enzymes), and the effects and routes of excretion of the metabolites of the drug.37
Risk-of-bias assessment categories:
Low risk of bias within a study design: These studies have the least bias and results are considered valid. There is a clear description of the population, setting, interventions, and comparison groups; appropriate measurement of outcomes; appropriate statistical and analytic methods and reporting; no reporting errors; low dropout rate; and clear reporting of dropouts.
Moderate risk of bias within a study design: These studies are susceptible to some bias, but it is not sufficient to invalidate the results. They do not meet all the criteria required for a rating of good quality because they have some deficiencies, but no flaw is likely to cause major bias. The study may be missing information, making it difficult to assess limitations and potential problems.
High risk of bias within a study design: These studies have significant flaws that imply biases of various types that may invalidate the results. They have serious errors in conduct, analysis, or reporting; large amounts of missing information; or discrepancies in reporting.
Strength of evidence grades35:
High: high confidence that the evidence reflects the true effect. Further research is very unlikely to change our confidence in the estimate of effect.
Moderate: moderate confidence that the evidence reflects the true effect. Further research may change our confidence in the estimate of effect and may change the estimate.
Low: low confidence that the evidence reflects the true effect. Further research is likely to change the confidence in the estimate of effect and is likely to change the estimate.
Insufficient: evidence either is unavailable or does not permit a conclusion.
Summary of Protocol Amendments
The review as planned (submitted protocol) was proven to require resources beyond those allocated to the EPC centre since the search of included data bases resulted in 32,000+ records. The EPC in agreement with AHRQ moved to perform a 10% title, abstract and full text screening to establish an estimation of the final size of the review (Dec2010-Jan 2011). The projected number of included studies based on this exercise was found to be beyond what is considered feasible for this systematic review.
Eligible Dietary Supplements
Upon mapping the findings of the scoping exercise, a second TEP call was arranged to seek expert advice regarding limiting the eligibility of included dietary supplements on Feb 23,, 2011. The EPC, TEP, AHRQ task order officer, and the clinical leads reached an agreement on March 11, 2011 (table 3). Based on this agreement the list on included supplements was restricted to those known to be commonly used in adults and elderly taking cardiovascular medication for which current evidence on possible drug-supplement interaction is lacking.
Limiting the Scope—Elimination of Key Question 5
To further optimize the allocated resources in producing evidence on clinical, surrogate, harms, and interaction outcomes on the most important dietary supplements known to be used by adults and elderly taking cardiovascular medication, it was further decided to eliminate the key question 5.
Eligible Languages
As per the protocol, the decision around language as an eligibility criterion would need to be made following initial screening in consultation with the task order officer.
Rational
The initial 10% screening (title and abstract) found 5 articles in German, 3 in Chinese, and 2 in Russian to be relevant to this review. Including studies in languages other than English is recommended for CAM topics.1,2 However, there is no clear guidance as to which languages to include. The Cochrane Handbook states “While the potential impact of studies published in languages other than English in a meta-analysis may be minimal, it is difficult to predict in which cases this exclusion may bias a systematic review. Review authors may want to search without language restrictions and decisions about including reports from languages other than English may need to be taken on a case-by-case basis”. Furthermore, the topic of dietary supplements added to conventional cardiovascular medications is not truly a CAM topic. Therefore, our EPC proposes to limit inclusion criteria to studies published only in the English language or German.
In the points below, we provide the multiple considerations for our proposal:
- Poor/uncertain applicability of evidence – studies published in the Chinese and even Russian language will unlikely be in practice settings reflective of developed countries, especially North America.
- There is evidence that randomized controlled trials published in some Chinese journals lacked an adequate description of randomization. Many non-randomized controlled trials were published as randomized controlled trials.3
- Except for CAM interventions like acupuncture and Tai Chi, there is no evidence or guidance recommending inclusion of Chinese language studies along with English.
- There is evidence that authors are more likely to report negative findings in a local journal. This was demonstrated for the German-language literature.4
| Item | Amendment | Section of the report in question | Date of proposed changes | Date effective |
|---|---|---|---|---|
| †Arjuna bark; Ashwagandha; B vitamins (pantothenic acid (B5), B6, folic acid (B9), B12); Calcium; Copper; Chromium; Iron; Potassium; Selenium; Ashwagandha; Butcher's Broom; Cinnamon; Cocoa; Caffeine; Danshen; Dandelion; Ephedra or Ma huang; Feverfew; Flax seed; Forskolin; Goji berry; Green tea; Guggulipid; Gymnema; Horse Chestnut; Licorice root; Lily of the Valley; Motherwort; Milk Thistle; Peppermint; Fo-ti; Pomegranate; Rauwolfia; Saw Palmetto; Soy; St. John’s wort; Turmeric; Yohimbe ; Valerian; Glucosamine; Olive oil; Alpha lipoic acid; Carotenoids; D-ribose; L-arginine; L-carnitine; L-glutamine; L-tryptophan; N-acetylcysteine; Plant sterols or stanols; Pycnogenol; Soy isoflavones; Taurine combinations of supplements (for example multivitamins, antioxidants, etc.) | ||||
| Revision to the list of dietary supplements | Include only:
|
Definition of intervention Method: Table 1 (intervention KQ 1- 4) |
March 03 | March 11 |
| Language of publication | Restriction to English and German for experimental and observational studies* | Methods: criteria for inclusion; Table 1: report characteristics | March 30 | April 04 |
| Restriction of key questions | Elimination of key question 5 | Key Questions: kQ5 Analytic framework Methods: criteria for inclusion; table 1 Grading the evidence |
March 03 | March 11 |
Review of Key Questions
For all EPC reviews, key questions were reviewed and refined as needed by the EPC with input from Key Informants and the Technical Expert Panel (TEP) to assure that the questions are specific and explicit about what information is being reviewed. In addition, for Comparative Effectiveness reviews, the key questions were posted for public comment and finalized by the EPC after review of the comments.
Key Informants
Key Informants are the end users of research, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making health care decisions. Within the EPC program, the Key Informant role is to provide input into identifying the Key Questions for research that will inform healthcare decisions. The EPC solicits input from Key Informants when developing questions for systematic review or when identifying high priority research gaps and needed new research. Key Informants are not involved in analyzing the evidence or writing the report and have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism
Key Informants must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their role as end-users, individuals are invited to serve as Key Informants and those who present with potential conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Technical Experts
Technical Experts comprise a multi-disciplinary group of clinical, content, and methodologic experts who provide input in defining populations, interventions, comparisons, or outcomes as well as identifying particular studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicted opinions are common and perceived as health scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, design and/or methodological approaches do not necessarily represent the views of individual technical and content experts. Technical Experts provide information to the EPC to identify literature search strategies and recommend approaches to specific issues as requested by the EPC. Technical Experts do not do analysis of any kind nor contribute to the writing of the report and have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism
Technical Experts must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals are invited to serve as Technical Experts and those who present with potential conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Peer Reviewers
Peer reviewers are invited to provide written comments on the draft report based on their clinical, content, or methodologic expertise. Peer review comments on the preliminary draft of the report are considered by the EPC in preparation of the final draft of the report. Peer reviewers do not participate in writing or editing of the final report or other products. The synthesis of the scientific literature presented in the final report does not necessarily represent the views of individual reviewers. The dispositions of the peer review comments are documented and will, for CERs and Technical briefs, be published three months after the publication of the Evidence report.
Potential Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Invited Peer Reviewers may not have any financial conflict of interest greater than $10,000. Peer reviewers who disclose potential business or professional conflicts of interest may submit comments on draft reports through the public comment mechanism.
Reference List
- Agency for Healthcare Research and Quality. Methods guide for effectiveness and comparative effectiveness reviews[Draft]. AHRQ Publication No. 10(11)-EHC063-EF. Available at: /sites/default/files/methods-guide_prepublication-draft_03-2011.pdf. Rockville, MD: AHRQ; 2011.
- Higgins J, Green S. Language bias. In: Cochrane handbook for systematic reviews of interventions, Wiley Online Library; 2008. Section 10.2.2.4. Available at: http://www.igh.org/Cochrane/tools/Ch10_Reporting.pdf.
- Wu T, Li Y, Bian Z, et al. Randomized trials published in some Chinese journals: how many are randomized? Trials 2009;10:46 . [PMID: 19573242].
- Egger M, Zellweger-Zahner T, Schneider M, et al. Language bias in randomised controlled trials published in English and German. Lancet 1997 Aug 2;350(9074):326-9. [PMID: 9251637].



