On August 25, 2011, amendments were made to this protocol. To view these amendments, please see the section titled “Summary of Protocol Amendments.”
Background and Objectives for the Systematic Review
Background
The World Health Organization (WHO) defines a health care-associated infection (HAI) as “…an infection occurring in a patient during the process of care in a hospital or other health care facility which was not present or incubating at the time of admission.”1 The Centers for Disease Control and Prevention (CDC) estimates that there were 1.7 million HAIs and 99,000 HAI-associated deaths in U.S. hospitals in 2002.2 The four largest categories of HAIs, responsible for over 80 percent of all HAIs, are catheter-associated urinary tract infections (32 percent), surgical site infections (22 percent), ventilator-associated pneumonia (15 percent), and central line-associated bloodstream infections (14 percent).2
The total annual direct medical costs of HAIs for U.S. hospitals ranges from $35.7 billion to $45 billion, adjusted to 2007 dollars using the consumer price index for inpatient hospital services.3 Using the same adjustment, the estimated patient hospital costs for the four most common HAIs are $3.45 billion to $10.07 billion for surgical site infections, $0.67 billion to $2.68 billion for central line-associated bloodstream infections, $1.03 billion to $1.50 billion for ventilator-associated pneumonia, and $0.39 billion to $0.45 billion for catheter-associated urinary tract infections.3
Less information is available on HAIs in nonhospital health care settings, such as ambulatory surgery centers, free-standing dialysis centers, and long-term care facilities such as nursing homes, but it is clear that HAIs are found in these settings as well and deserve attention (see, e.g., Anand and Kollef,4 CDC,5 Edmunston and Foulkes,6 Ouslander et al.7). Although comparable estimates of the costs of HAIs for nonhospital settings were not found, the total direct medical costs of HAIs would clearly be higher if other settings were included.
Much of the effort devoted to reducing HAIs has focused on their prevention. According to the CDC, about one in four patients who acquire a bloodstream infection from the insertion of a central line dies.8 Identifying more effective ways to treat these patients is essential, but it is far better to prevent the infections in the first place. The prevention and reduction of HAIs is a top priority for the U.S. Department of Health and Human Services.9 A CDC report estimated that the cost savings of preventing 70 percent of HAIs would be $25.0 billion to $31.5 billion, adjusted using the consumer price index for inpatient hospital services.3
There are a number of steps to be taken in creating an evidence base that supports efforts to prevent HAIs. Once the targets of the efforts are identified—for example, the types of infections and the most common locations or patient populations—the next step is to identify preventive interventions that, if undertaken, are likely to prevent these HAIs. Extensive research in this area underpins evidence-based guidelines such as those produced by the Healthcare Infection Control Practices Advisory Committee (HICPAC) and jointly by the Society of Healthcare Epidemiologists of America and the Infectious Diseases Society of America (SHEA/IDSA).
Knowledge of effective interventions to prevent HAIs can be difficult to convert into practice, however. The challenges of implementing prevention and surveillance programs for HAIs are many. These efforts are labor intensive and require an infrastructure that coordinates the education and supervision of staff and, in larger health care facilities, computerized support.10 As extensive efforts to improve compliance with hand washing have shown, it can be difficult to translate the knowledge that hand washing can reduce infections into consistent, appropriate practice. The implementation of quality improvement strategies for hand washing remains less developed, although recognition of its importance continues to increase.
For these reasons, how to spur the adoption of preventive interventions has been the subject of considerable research in recent years. Some efforts have been initiated internally by dedicated staff, while others have been spurred in part by external forces,11 for example, the Medicare policy that does not allow payment for “never events,” which include “[p]atient death or serious disability associated with the use of contaminated drugs, devices or biologics provided by the healthcare facility.”12 These efforts are taking place in single hospitals or in units within a hospital, as well as across entire States and even between multiple States. For example, the Agency for Healthcare Research and Quality (AHRQ) has funded the Comprehensive Unit-based Safety Program (CUSP)13 to disseminate toolkits developed for the Michigan Keystone ICU [Intensive Care Unit] Project14 to every other State, the District of Columbia, and Puerto Rico.. The focus will first be on bloodstream infections and then on catheter-associated urinary tract infections. An interim report released in April 2011 indicated a 35 percent reduction in central line-associated bloodstream infection rates from 1.8 to 1.17 infections per 1,000 central line days among 350 adult ICUs.15
In 2007, AHRQ published an Evidence Report/Technology Assessment (hereafter, Evidence Report) on the prevention of HAIs16 as Volume 6 of Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. The objective of that Evidence Report was to identify quality improvement strategies that successfully increase adherence to effective preventive practices and reduce infection rates for the following HAIs:
- Surgical site infection (SSI)
- Central line-associated bloodstream infection (CLABSI)
- Ventilator-associated pneumonia (VAP)
- Catheter-associated urinary tract infection (CAUTI)
The Evidence Report concluded that preliminary data indicated that several strategies were worthy of further study and possibly wider implementation and that higher quality improvement studies of implementation were needed. The volume and range of activity in this field since 2007 warrants an update and re-examination of what has been learned about how to effectively implement strategies we know work.
Objective
Our systematic review will update and expand, where warranted, the 2007 Evidence Report.16 The objective of our evidence review is to also identify quality improvement strategies that successfully increase adherence to effective preventive practices and reduce infection rates for SSIs, CLABSIs, VAP, and CAUTIs. Successful strategies will help to close the quality gap regarding health care-associated infections.
Scope
Our review will evaluate the large number of implementation studies that have been published since 2006, when the literature search for the 2007 Evidence Report ended. Like the 2007 report, our review will focus on the implementation of preventive interventions that are recommended for universal use in target patient populations by professional societies and governmental organizations. The CDC guidelines (http://www.cdc.gov/hicpac/pubs.html) are developed by HICPAC, which was formed to provide guidance to the CDC and the Secretary of Health and Human Services regarding strategies for prevention and surveillance of HAIs, and includes broad stakeholder input.17 In addition to the CDC guidelines, we reviewed the SHEA/IDSA Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals (hereafter, Compendium).18 The SHEA/IDSA Compendium highlights evidence-based HAI prevention strategies to be implemented in acute care hospitals.
In planning our evidence review, we assessed whether the topic was still relevant and whether changes in scope were warranted.Given the continued prevalence of these infections despite efforts to reduce them, we found that the topic remains relevant and that many studies have been published since 2007. Whether the list of infections should be expanded was also considered, specifically three possible additions: methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, and norovirus. MRSA and C. difficile are currently the subjects of ongoing AHRQ-funded comparative effectiveness reviews whose protocols are available at the Effective Health Care Program Web site (see /sites/default/files/mrsascreening_protocol_20110602.pdf and /sites/default/files/pdf/c-diff-infections_research-protocol.pdf). The study on MRSA focuses specifically on the effectiveness of universal screening, while the report on C. difficile is broader in scope. Serious consideration was given to the inclusion of norovirus, because the need to include cleaning staff, clinical staff, and others in efforts to reduce infections might shed more light on the importance of context in influencing the effectiveness of various quality improvement (QI) strategies to implement preventive interventions. However, the HICPAC guidelines for norovirus were in draft form when our project started.Furthermore, there was concern that the epidemic nature of norovirus outbreaks would make it more difficult to link specific QI strategies to changes in infection rates. Based on these factors, the same list of four infections from the 2007 Evidence Report will be used.
The scope of our review was expanded in another manner by broadening the list of health care settings included. Much of the initial work on HAIs focused on hospital settings, as is evident in the 2007 Evidence Report. Since that time, there has been increased recognition of the importance of efforts to reduce HAIs in other health care settings, such as ambulatory surgery centers, free-standing dialysis centers, and long-term care facilities.19 Our review, therefore, will include studies on the effectiveness of QI strategies in these nonhospital health care settings as well.
In our review we also will apply the recommendations, where applicable, of the report RAND Health prepared for AHRQ.20 The objective of the RAND Health report was to identify criteria for assessing the impact of context on the effectiveness of patient safety practices, which are a type of quality improvement strategy. The context of an intervention—for example, the type of health care setting, the leadership structure, the safety culture, and openness to innovation—can have an important impact on whether preventive interventions are adopted. Furthermore, the ability to transfer a successful QI strategy from one setting to another may depend in part on whether the contexts differ. For example, cultures in which nurses are able to question a physician’s adherence to recommended practices may be able to implement an intervention more successfully than those where they are not. In the RAND Health report, Shekelle et al.20 noted that context may be the distinguishing factor between evaluating the impact of a quality improvement strategy versus a clinical intervention. The RAND Health report identified important evaluation questions for QI strategies, reporting requirements, and the elements of context that may have an impact on the effectiveness of implementing QI strategies (Appendix 1), all of which are incorporated into our proposed evidence review.
Terminology Used in Our Review
- The first step in designing a quality improvement project is to identify a quality gap. According to Ranji et al.,16 a quality gap refers to the difference between health care processes or outcomes observed in practice and those potentially achievable on the basis of current professional knowledge. In this proposal, the quality gaps are the levels of HAIs found in various health care settings.
- A quality improvement target is the outcome process or structure that the QI strategy is aimed at changing (adapted from Ranji et al.16).
- A preventive intervention is a specific infection-control practice that has been demonstrated to reduce the incidence of a HAI.16 An example would be using maximal sterile barrier precautions when inserting a central line.
- A quality improvement strategy aims to narrow the quality gap for a group of patients who are representative of those seen in routine practice by increasing the use of preventive interventions (adapted from Ranji et al.16). An example would be staff training about the use of maximal sterile barrier precautions and authorizing any member of the team to stop the procedure if any part(s) of the preventive intervention is not being used.
- The effectiveness of a quality improvement strategy is dependent not only on the strategy chosen but also on the context in which it is implemented. According to the RAND Health report,20 there is no standard definition of context but it may include barriers and facilitators related to the organizational and policy environment, as well as information about the processes of implementation.
The Key Questions
Question 1
Which quality improvement strategies are effective in reducing the following health care-associated infections?
- Surgical site infections (SSIs)
- Central line-associated bloodstream infections (CLABSIs)
- Ventilator-associated pneumonia (VAP)
- Catheter-associated urinary tract infections (CAUTIs)
Both benefits and unintended consequences should be examined, as well as the duration of any effect. Quality improvement strategies include the following:
- Clinician education
- Patient education
- Audit and feedback
- Clinician reminder systems
- Organizational change
- Financial or regulatory incentives for patients or clinicians
- A combination of the above
- Which quality improvement strategies (see the bulleted list of strategies above) are effective in increasing adherence to evidence-based preventive interventions for the four health care-associated infections listed above?
A list of specific, evidence-based preventive interventions for each type of infection—based on the HICPAC guidelines (http://www.cdc.gov/hicpac/pubs.html), the SHEA/IDSA Compendium,18 and hand hygiene in general—will be used to select relevant studies. - What is the cost, return on investment, or cost-effectiveness for health care providers, patients, and society as a whole of quality improvement strategies to reduce the four health care-associated infections (SSIs, CLABSIs, VAP, and CAUTIs)?
The response to this question should be based on a synthesis of the available literature and not on the development of a new cost-effectiveness analysis. - Which factors are associated with the effectiveness of quality improvement strategies, including, for example:
- Which quality improvement strategies (see the bulleted list of strategies above) are effective in increasing adherence to evidence-based preventive interventions for the four health care-associated infections listed above?
- Type of quality improvement strategy (see the bulleted list of strategies above)
- Duration of intervention
- Setting, for example, hospitals (ICU, surgical, or ventilator-dependent patients), outpatient surgical centers, long-term care facilities, and free-standing dialysis centers
- Kinds of clinicians who implement the quality improvement strategies
Question 2
What is the impact of the health care context on the effectiveness of quality improvement strategies, including reduced infections and increased adherence to preventive interventions?
The context may include structural organizational characteristics; external factors, for example, public mandatory reporting; patient safety culture, teamwork, leadership at the unit level; and availability of implementation and management tools.
PICOTS Criteria
The following table outlines the populations, interventions, comparators, outcomes, timing, and settings of interest (PICOTS) in our report:
| PICOTS Element | |
|---|---|
| Abbreviations: CAUTI = catheter-associated urinary tract infection; CDC = Centers for Disease Control and Prevention; CLABSI = central line-associated bloodstream infection; HICPAC = the Healthcare Infection Control Practices Advisory Committee; IDSA = Infectious Diseases Society of America; SHEA = Society of Healthcare Epidemiologists of America; SSI = surgical site infection; VAP = ventilator-associated pneumonia | |
| Population |
Patients in selected health care settings (listed under “Settings”); clinicians caring for these patients; and health care institutional leadership
Subpopulations of particular interest include the following:
|
| Intervention | Quality improvement strategies to reduce SSIs, CLABSIs, VAP, and CAUTIs by increasing use of evidence-based infection-prevention practices identified by the CDC, HICPAC, or SHEA/IDSA (see Appendix 2) |
| Comparator | Usual practice or alternative strategies |
| Outcomes |
Primary outcomes:
|
| Timing | Before and after the intervention, with followup to gauge the durability of the effect (minimum of 1 year) |
| Setting |
|
Analytic Framework
Figure 1. Analytical framework for the systematic review on quality improvement strategies to reduce health care–associated infections*
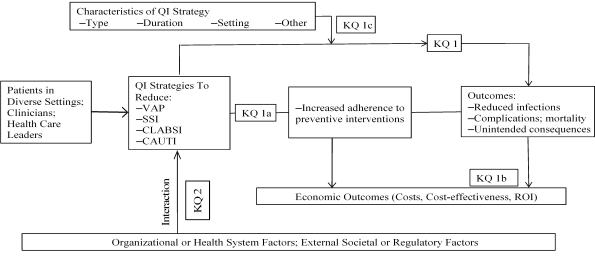
*Adapted from Appendix H in Shekelle et al.20
Abbreviations: CAUTI = catheter-associated urinary tract infection; CLABSI = central line–associated bloodstream infection; KQ = key question; QI = quality improvement; ROI = return on investment; SSI = surgical site infection; VAP = ventilator-associated pneumonia
Methods
Our review will follow, where feasible, the recommendations in the RAND Health report.20 The authors of the report recommend three evaluation questions for patient safety practices (PSPs), which in our case will be applied to quality improvement strategies aimed at increasing adherence to preventive interventions to reduce HAIs:
- What is the effectiveness of the QI strategy (effectiveness and harms)?
- What is the implementation experience of the quality improvement strategy at individual institutions?
- What is the success of widespread adoption, spread, and sustainability of the quality improvement strategy?
The specific prevention interventions used to reduce infections will be selected from recommendations with a grade of 1A or 1B in the HICPAC guidelines,21 which is analogous to the approach used in the 2007 Evidence Report,16 or with a grade of A-I or A-II in the SHEA/IDSA Compendium.18 A list of such preventive interventions, as reviewed and amended by our Technical Expert Panel, is found in Appendix 2. Our review, in turn, will focus on the effectiveness of quality improvement strategies and how these efforts are affected by the context in which they are implemented.Appendix 3 lists the elements recommended by the authors of the RAND Health report20 for reporting on the implementation of PSPs, which apply in our review to the quality improvement strategies, as well as the four high-priority contexts.
A. Criteria for Inclusion/Exclusion of Studies in the Review
The same selection criteria will be used for our review as for the 2007 Evidence Report,16 with the addition of a criterion related to the setting. Specifically, included studies will be required to:
- Report the effect of an intervention on the incidence of health care-associated infections (CAUTIs, CLABSIs, SSIs, or VAP) or report the effect of an intervention on adherence to evidence-based prevention interventions. A list of such preventive interventions is found in Appendix 2. Our Technical Expert Panel was consulted about the list of measures.
- Use either an experimental design with a control group (randomized or quasi-randomized controlled trial, controlled before-after study) or a quasi-experimental design (interrupted time series or simple before-after study). Quasi-experimental studies must have a clearly defined intervention time period; interrupted time series designs must report at least three time points of data before and after the intervention.
- Report on one of the following settings: hospitals, outpatient surgical centers, free-standing dialysis centers, and long-term care facilities.
- To be included, trials that report related outcomes (e.g., costs, health services utilization, patient or provider satisfaction with care) or unanticipated consequences of an intervention must also report infection rates or adherence with preventive interventions.
- Articles will be limited to the English language. The interventions evaluated in this report are heavily dependent on the health care context, and therefore non-English studies are unlikely to add much applicable data. While English-language articles from all countries will be included, differences in the organization of health care outside of the United States may limit the applicability of those findings.
B. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies To Answer the Key Questions
The same search strategy will be used in our review as in the prior Evidence Report16 (see Appendix 4). The search will be rerun in the MEDLINE, CINAHL, and EMBASE databases. Duplicate records will be deleted. The search will cover January 2006, when the search for the last Evidence Report ended, to the present. The search will be updated while the draft report is available for public comment, and relevant articles will be added. Additional efforts will be made to identify articles on interventions in nonhospital settings, which are likely to be reported less frequently. This step will include queries of the members of the Technical Expert Panel and of individuals the Panel recommends we contact as experts on these intervention settings.. We will also screen the bibliographies of included articles to identify additional references. Web sites of entities involved in efforts to reduce HAIs, such as the Institute for Healthcare Improvement, will be scanned to ensure that no relevant peer-reviewed publications were missed and to identify descriptions of implementation strategies for which outcomes have been published in the peer-reviewed literature.
The titles and abstracts from the literature search results will be screened, and each citation will be marked as: 1) eligible for full-text abstraction; 2) ineligible for full-text abstraction; or 3) uncertain. Studies will be marked as eligible for full-text abstraction if the citation reports the outcomes of an intervention for a) any one of the four specified HAIs or b) a combination of HAIs that include at least one of the four. Synonyms for the HAI-related terms are found in section VI.
The reasons for excluding an article will be noted. Articles deemed uncertain for full-text abstraction will be reviewed by a second reviewer. Any disagreements will be resolved through discussion; if necessary, a third reviewer will be brought in to resolve the issue.
Once full-text articles are retrieved, a process similar to that used for the review of titles and abstracts will be followed to select the final group of articles for inclusion in our review. Articles will be included if they report on the implementation of one of the preventive interventions to reduce one or more of the four HAIs covered in our review. A list of such preventive interventions is found in Appendix 2. The Technical Expert Panel will be consulted about the list of measures.
C. Data Abstraction and Data Management
A draft list of data elements is found in Appendix 5. Those articles selected for inclusion in our review will undergo abstraction by a single reviewer; a second reviewer will “fact check” the abstracted items, using a clean copy of the article. Any discrepancies will be resolved by discussion or the inclusion of a third reviewer, if necessary. To increase consistency across abstractors, each one will independently abstract the several articles at the beginning of the abstraction process. A meeting will be held to discuss any differences and to reach consensus on common strategies. If a new abstractor is added later, he or she will abstract the same five articles and then be informed about the common strategies. Quality appraisals for each article will be developed independently by two reviewers; discrepancies will be resolved by discussion or by the inclusion of a third reviewer if necessary.
As recommended by the RAND Health report,20 data will be collected on the context, the intervention program, and the interaction between the two, where available. Changes in the intervention over the course of implementation will also be noted.
D. Assessment of Methodological Quality of Individual Studies
To assess the quality of the studies included in our review, we will utilize the quality assessment criteria developed by the authors of the 2007 AHRQ Evidence Report on health care-associated infections.16 Due to the nature of the literature on this topic, qualitative rather than quantitative assessments will be made. As in the prior Evidence Report, studies meeting both criteria for external validity and two of three criteria for internal validity will be considered to have stronger internal and external validity.
Determining Internal Validity for Quasi-experimental Studies
The quality improvement literature consists mainly of quasi-experimental or simple before-after studies, both of which have limitations when attributing causality to an intervention. In quasi-experimental studies of infection control, the following may result in the attribution of causality to factors other than the intervention: 1) difficulty in controlling for confounding variables, 2) regression to the mean, and 3) maturation effects and secular trends affecting baseline and/or postintervention measurements.22 The following criteria will be used to assess internal validity, within the limitations of the study design, and to identify studies in which causality could be attributed to the intervention.
- Was the intervention performed independent of other QI efforts or other changes?
If other quality improvement efforts were being implemented simultaneously in the same health care setting, they may impact the quality improvement efforts directed specifically at prevention of HAIs. - Did the study report data at more than one time point before and after the intervention?
Reporting more than one data point before the intervention can affirm consistency in the HAI rate or indicate a rising or falling rate before the intervention. Reporting more than one data point after the intervention can indicate sustainability of the intervention. An interrupted time series design, which requires three time points of data both before and after the intervention at a minimum, can better determine a true intervention effect. - If the study reported infection rates, were process measurements also reported?
Providing data on adherence to the intervention strategies provides complementary information to the infection rates. If studies report that infection rates decreased and adherence rates increased, this provides indirect support that the intervention was effective. Also, adherence measurements do not have to be adjusted for a patient’s underlying risk of infection and therefore can be compared across hospitals and studies.23 Reporting of adherence measures can thus improve both the internal and external validity of the study.
Determining Internal Validity for Controlled Studies
- Method of treatment assignment
- Were study subjects randomized, and if so, was the randomization process described?
- For nonrandomized studies, was the rationale for selection of the comparison group explained, and was a baseline observation period included (to assess selection bias)?
- Blinding
- Were the outcome assessors blinded to treatment group assignment?
- Statistical analysis
- Was a unit-of-analysis error present? If so, were appropriate statistical methods used for correction?
In appraising the quality of a study, the relative merits of the study design will also be considered. For example, studies with a control group are generally of higher quality than are those with no control group. Similarly, studies with multiple measures of outcomes both before and after a strategy is implemented will provide more credible results.
Questions 12, 14, 15, 17, 24, and 29 from the RTI Item Bank24 for assessing risk of bias and precision for observational studies will also be added where appropriate. They are as follows:
- Did the execution of the study vary from the original protocol?
- Is the intervention assessed using valid and reliable measures, implemented consistently across all study participants?
- Are outcomes assessed using valid and reliable measures, implemented consistently across all study participants?
- Is the length of followup sufficient to support the evaluation of primary outcomes and harms?
- Are any important primary outcomes missing from the results?
- Is the source of funding identified?
Determining External Validity for All Studies
Studies reporting adherence measures will be considered to have greater external validity. The following questions will be used to assess external validity for studies reporting infection rates.
- If the study reported infection rates, did the study use CDC/National Healthcare Safety Network (NHSN) methodology for measuring infections?
The NHSN definitions for nosocomial infections are the accepted standard.25 - For CLABSIs, VAP, and CAUTIs: If the study reported infection rates, were those reported rates adjusted for device utilization?
HAI rates should be adjusted for potential risk factors. A basic level of risk stratification, according to the NHSN, is to report rates as infections per 1,000 device-days. - For SSI: If the study reported infection rates, was surveillance for infections performed after hospital discharge?
Depending on the surgical procedure, a large proportion of infections may occur after discharge from the hospital. If postdischarge surveillance was not conducted, SSI rates can be underestimated.
E. Data Synthesis
The primary outcome is a reduction in infection rates, since without that outcome further examination of the quality improvement strategies may not be useful. At the same time, however, the key questions facing many health care institutions today are: 1) How can these interventions be implemented? and 2) How does context affect implementation efforts? The primary focus of our review will be on these latter issues, which are highlighted in the RAND Health report.20 The results of higher quality studies will be emphasized. The evidence will be summarized in a narrative format. The heterogeneity expected in the quality improvement targets (i.e., the types of infections), quality improvement strategies, prevention interventions, contexts, and study designs is likely to preclude a meta-analysis. The results will be compiled according to type of infection, but an effort will be made to identify common findings. The subgroups that will be examined are defined in the PICOTS table and in the Key Questions at the beginning of this document.
F. Grading the Evidence for Each Key Question
The GRADE criteria as modified by AHRQ26 and by the authors of the RAND Health report for patient safety practices (Chapter 13 in Shekelle et al.20; also see Appendix 6 below) will be used. Conducting randomized controlled trials on this topic with sufficient sample size (e.g., if randomized by location) is difficult, and many observational studies may be included in our review. The Technical Expert Panel will be consulted on how the GRADE criteria should be applied in this case and what additional explanation, if any, may be useful.
G. Assessing Applicability
As made clear in the RAND Health report,20 the context in which strategies are implemented may have a large impact on their effectiveness. The project team will collect any data available on context and implementation (see the draft list of data elements in Appendix 5). In assessing the applicability of QI strategies, their use and effectiveness in different health care settings will be evaluated to aid end users.
References
- World Health Organization. The burden of health care-associated infection worldwide. Available at: http://www.who.int/gpsc/country_work/burden_hcai/en/index.html. Accessed July 29, 2011.
- Klevens RM, Edwards JR, Richards CL, Jr., et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007 Mar-Apr;122(2):160-6. PMID: 17357358.
- Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Atlanta, GA: Centers for Disease Control and Prevention; March 2009. Available from: http://www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Last accessed July 2011.
- Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP, and VAP. Semin Respir Crit Care Med 2009 Feb;30(1):3-9. PMID: 19199181.
- Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011 Mar 4;60(8):243-8. PMID: 21368740.
- Edmonston DL, Foulkes GD. Infection rate and risk factor analysis in an orthopaedic ambulatory surgical center. J Surg Orthop Adv 2010 Fall;19(3):174-6. PMID: 21086932.
- Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc 1987 Dec;35(12):1063-70. PMID: 3680838.
- Centers for Disease Control and Prevention. Vital Signs. Making Health Care Safer: Reducing Bloodstream Infections. Available at: http://www.cdc.gov/VitalSigns/HAI/index.html. Accessed July 29, 2011.
- U.S. Department of Health and Human Services. HHS.gov. HHS Action Plan To Prevent Healthcare-Associated Infections. Available at: http://www.hhs.gov/ash/initiatives/hai/infection.html. Accessed July 29, 2011
- Yokoe DS, Classen D. Improving patient safety through infection control: a new healthcare imperative. Infect Control Hosp Epidemiol 2008 Oct;29 Suppl 1:S3-11. PMID: 18840086.
- National Quality Forum. Serious Reportable Events. Available at: http://www.qualityforum.org/Publications/2008/10/Serious_Reportable_Events.aspx. Accessed July 29, 2011.
- Centers for Mediare and Medicaid Services. MA [Medicare Advantage] Payment Guide for Out of Network Payments. 6/01/2011 Update. Available at: http://www.cms.gov/MedicareAdvtgSpecRateStats/downloads/oon-payments.pdf. Accessed July 28, 2011.
- Agency for Healthcare Research and Quality. Fact Sheet: Ending Healthcare-Associated Infections. October 2009. AHRQ Publication No. 09(10)-P013-2. Available at: http://www.ahrq.gov/qual/haicusp.pdf. Accessed July 29, 2011.
- Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstrem infections in the ICU. N Engl J Med 2006 Dec 28;355(26):2725-32. PMID: 17192537.
- Health Research and Educational Trust, Johns Hopkins University Quality and Safety Research Group, and Michigan Health and Hospital Association Keystone Center for Patient Safety and Quality. Eliminating CLABSI: A National Patient Safety Imperative: A Progress Report on the National On the CUSP: Stop BSI Project. Rockville, MD: Agency for Healthcare Research and Quality; April 2011. AHRQ Publication No. 11-0037-EF. Available at: http://www.ahrq.gov/qual/onthecusprpt/onthecusp.pdf. Accessed July 29, 2011.
- Ranji SR, Shetty K, Posley KA, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Volume 6—Prevention of Healthcare–Associated Infections. Evidence Report/Technology Assessment No. 9 (Prepared by Stanford University–UCSF Evidence-based Practice Center under Contract No. 290-02-0017). Rockville, MD: Agency for Healthcare Research and Quality; January 2007. AHRQ Publication No. 04(07)-0051-6. Available AT: http://www.ncbi.nlm.nih.gov/books/NBK43982/pdf/TOC.pdf. Accessed July 29, 2011.
- Centers for Disease Control and Prevention. Healthcare Infection Control Practices Advisory Committee (HICPAC). About HICPAC. Available at: http://www.cdc.gov/hicpac/about.html. Accessed July 29, 2011.
- Society for Healthcare Epidemiology of America and Infectious Diseases Society of America. Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals. Available at: http://www.shea-online.org/GuidelinesResources/CompendiumofStrategiestoPreventHAIs.aspx. Accessed July 29, 2011.
- Centers for Disease Control and Prevention. Healthcare-associated Infections (HAIs). Guide to Infection Prevention for Outpatients Settings: Minimum Expectations for Safe Care. May 2011. Available at: http://www.cdc.gov/HAI/settings/outpatient/outpatient-care-guidelines.html. Accessed July 29, 2011.
- Shekelle PG, Pronovost PJ, Wachter RM, et al, and the Patient Safety Practices Technical Expert Panel. Assessing the Evidence for Context-Sensitive Effectiveness and Safety of Patient Safety Practices: Developing Criteria. Contract Final Report (Prepared by RAND Health under Contract No. HHSA-290-2009-10001C). Rockville, MD: Agency for Healthcare Research and Quality; December 2010. AHRQ Publication No. 11-0006-EF. Available at: http://www.ahrq.gov/qual/contextsensitive/context.pdf. Accessed July 29, 2011.
- Centers for Disease Control and Prevention. Healthcare Infection Control Practices Advisory Committee (HICPAC). General Guidelines. Available at: http://www.cdc.gov/hicpac/pubs.html. Accessed July 29, 2011.
- Harris AD, Bradham DD, Baumgarten M, et al. The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004 Jun 1;38(11):1586-91. PMID: 15156447.
- McKibben L, Horan T, Tokars JI, et al. Guidance on public reporting of healthcare-associated infections: recommendations of the Healthcare Infection Control Practices Advisory Committee. Am J Infect Control 2005 May;33(4):217-26. PMID: 15877016.
- Viswanathan M, Berkman ND. Assessing the Risk of Bias and Precision of Observational Studies of Intervention or Exposures: Development, Validation, and Reliability Testing of the RTI Item Bank. Prepared by RTI International–University of North Carolina Evidence-based Practice Center under Contract No. 290200710056I. Rockville, MD: Agency for Healthcare Research and Quality; 2011 (in press).
- Emori TG, Edwards JR, Culver DH, et al. Accuracy of reporting nosocomial infections in intensive-care-unit patients to the National Nosocomial Infections Surveillance System: a pilot study. Infect Control Hosp Epidemiol 1998 May;19(5):308-16. PMID: 9613690.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol 2010 May;63(5):513-23. PMID: 19595577.
Definition of Terms
CAUTI = catheter-associated urinary tract infections (synonyms: urinary catheter–associated urinary tract infection, Foley catheter–associated urinary tract infection, urinary catheter–related infection, urinary catheter–associated cystitis)
CLABSI = central line–associated bloodstream infection (synonyms: central venous catheter–associated infection, central venous catheter sepsis, central line sepsis)
PSP = patient safety practice
SSI = surgical site infection (synonyms: surgical wound infection, postoperative infection)
VAP = ventilator-associated pneumonia
Summary of Protocol Amendments
In the event of protocol amendments, the date of each amendment will be accompanied by a description of the change and the rationale.
8/25/11. Section IV.C. , end of first paragraph, is changed as follows (changes are bolded): Quality appraisals for each higher quality articles will be developed independently by two reviewers; discrepancies will be resolved by discussion, or the inclusion of a third reviewer, if necessary. Higher quality articles exclude studies with simple before-after designs in which only t-tests or chi-square tests are used to evaluate the statistical significance of any change.
This protocol is being amended because studies using t-tests or chi-square tests are poor quality based on the research design and statistical tests used and can only be used for hypothesis generation. Their scores on other quality metrics will not change that assessment. Furthermore, determining the research design and statistical tests used is straightforward and does not entail a subjective assessment, as quality metrics may require. Therefore, fact checking of the quality appraisals for these low quality studies will be performed instead of double, independent assessment.
Technical Experts
Technical Experts comprise a multi-disciplinary group of clinical, content, and methodological experts who provide input in defining populations, interventions, comparisons, or outcomes as well as identifying particular studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicted opinions are common and perceived as health scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, design and/or methodological approaches do not necessarily represent the views of individual technical and content experts. Technical Experts provide information to the EPC to identify literature search strategies and recommend approaches to specific issues as requested by the EPC. Technical Experts do not do analysis of any kind nor contribute to the writing of the report.
Technical Experts must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals are invited to serve as Technical Experts and those who present with potential conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Peer Reviewers
Peer reviewers are invited to provide written comments on the draft report based on their clinical, content, or methodological expertise. Peer review comments on the preliminary draft of the report are considered by the EPC in preparation of the final draft of the report. Peer reviewers do not participate in writing or editing of the final report or other products. The synthesis of the scientific literature presented in the final report does not necessarily represent the views of individual reviewers. The dispositions of the peer review comments are documented and will, for CERs and Technical briefs, be published 3 months after the publication of the Evidence report.
Potential Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Invited Peer Reviewers may not have any financial conflict of interest greater than $10,000. Peer reviewers who disclose potential business or professional conflicts of interest may submit comments on draft reports through the public comment mechanism.
Appendix 1. Types of Quality Improvement Strategies
(Source: Ranji et al.16)

Appendix 2. Evidence-Based Preventive Interventions Used in Study Selection
The following evidence-based preventive interventions, identified from the 2007 Evidence Report (Ranji et al.16), the HICPAC guidelines,21 the SHEA/IDSA Compendium,18 and input from the Technical Expert Panel, were used for identifying studies from all settings for inclusion in this evidence review.
All HAIs: Hand hygiene
Surgical site infection:
- appropriate perioperative antibiotic prophylaxis (including appropriate antibiotic selection, timing, and duration) (2007 report)
- perioperative glucose control (2007 report)
- decreasing shaving [or hair removal] of the operative site (2007 report)
- specific technique for clinicians when washing hands prior to surgery (CDC/HICPAC IB)
- treat infections prior to surgery (CDC/HICPAC IA)
- encourage tobacco cessation (CDC/HICPAC IB)
- bathe and prepare skin with antiseptic agent (CDC/HICPAC IB)
- develop policies to manage infected surgical team (CDC/HICPAC IB)
- maintain positive pressure ventilation and minimal 15 air changes per hr during surgery (CDC/HICPAC IB)
- disinfect environmental surfaces (CDC/HICPAC IB)
- sterile instruments and surgical wear (CDC/HICPAC IB)
- after surgery, protect incision with sterile dressing (CDC/HICPAC IB)
- normothermia [recommended by Technical Expert Panel]
- intraoperative administration of oxygen (FIO2), for abdominal or colorectal cases [recommended by Technical Expert Panel]
Central line-associated bloodstream infection:
- adherence to maximal sterile barrier precautions (2007 report)
- use of chlorhexidine for skin antisepsis (2007 report); If there is a contraindication to chlorhexidine, tincture of iodine, an iodophor, or 70% alcohol can be used as alternatives. (CDC/HICPAC IA)
- avoidance of femoral catheterization (2007 report)
- decontaminate hands before donning sterile gloves when inserting a central intravascular catheter (CDC/HICPAC IB)
- do not use arterial or venous cutdown procedures during insertion (CDC/HICPAC IA)
- do not use organic solvents on skin (CDC/HICPAC IA)
- clean injection ports with 70% alcohol before accessing (CDC/HICPAC IA)
- prepare admixtures using sterile technique (CDC/HICPAC IB)
- do not use in-line filters for infection-control purposes (CDC/HICPAC IA)
- do not administer systemic antimicrobial prophylaxis routinely prior to catheter insertion (CDC/HICPAC IA)
- after insertion, remove nonessential catheters (SHEA/IDSA A-II); Promptly remove any intravascular catheter that is no longer essential (CDC/HICPAC IA)
- after insertion, change dressings and perform site care every 5-7 days and change gauze every 2 days (SHEA/IDSA A-I); Replace dressings used on short-term CVC sites at least every 7 days for transparent dressings, except in those pediatric patients in which the risk for dislodging the catheter may outweigh the benefit of changing the dressing. (CDC/HICPAC IB)
- after insertion, use antimicrobial ointments (SHEA/IDSA A-I); Do not use topical antibiotic ointment or creams on insertion sites, except for dialysis catheters, because of their potential to promote fungal infections and antimicrobial resistance. (CDC/HICPAC IB) (Need to resolve inconsistency based on TEP advice.)
- Weigh the risks and benefits of placing a central venous device at a recommended site to reduce infectious complications against the risk for mechanical complications (e.g., pneumothorax, subclavian artery puncture, subclavian vein laceration, subclavian vein stenosis, hemothorax, thrombosis, air embolism, and catheter misplacement) (CDC/HICPAC IA)
- Avoid the subclavian site in hemodialysis patients and patients with advanced kidney disease, to avoid subclavian vein stenosis (CDC/HICPAC IA)
- Use a fistula or graft in patients with chronic renal failure instead of a CVC for permanent access for dialysis (CDC/HICPAC IA)
- Use ultrasound guidance to place central venous catheters (if this technology is available) to reduce the number of cannulation attempts and mechanical complications. Ultrasound guidance should only be used by those fully trained in its technique. (CDC/HICPAC IB)
- Use a CVC with the minimum number of ports or lumens essential for the management of the patient. (CDC/HICPAC IB)
- When adherence to aseptic technique cannot be ensured (i.e., catheters inserted during a medical emergency), replace the catheter as soon as possible, i.e., within 48 hours. (CDC/HICPAC IB)
- Maintain aseptic technique for the insertion and care of intravascular catheters. (CDC/HICPAC IB)
- Antiseptics should be allowed to dry according to the manufacturer’s recommendation prior to placing the catheter. (CDC/HICPAC IB)
- Use either sterile gauze or sterile, transparent, semipermeable dressing to cover the catheter site. (CDC/HICPAC IA)
- Replace catheter site dressing if the dressing becomes damp, loosened, or visibly soiled. (CDC/HICPAC IB)
- Do not submerge the catheter or catheter site in water. Showering should be permitted if precautions can be taken to reduce the likelihood of introducing organisms into the catheter (e.g., if the catheter and connecting device are protected with an impermeable cover during the shower). (CDC/HICPAC IB)
- Ensure that catheter site care is compatible with the catheter material. (CDC/HICPAC IB)
- Monitor the catheter sites visually when changing the dressing or by palpation through an intact dressing on a regular basis, depending on the clinical situation of the individual patient. If patients have tenderness at the insertion site, fever without obvious source, or other manifestations suggesting local or bloodstream infection, the dressing should be removed to allow thorough examination of the site. (CDC/HICPAC IB)
- Do not routinely replace CVCs, PICCs, hemodialysis catheters, or pulmonary artery catheters to prevent catheter-related infections. (CDC/HICPAC IB)
- Do not use guidewire exchanges routinely for non-tunneled catheters to prevent infection. (CDC/HICPAC IB)
- Do not use guidewire exchanges to replace a non-tunneled catheter suspected of infection. (CDC/HICPAC IB)
- Use a guidewire exchange to replace a malfunctioning non-tunneled catheter if no evidence of infection is present. (CDC/HICPAC IB)
Ventilator-associated pneumonia:
- Semi-recumbent patient positioning (2007 report)
- daily assessment of readiness for ventilator weaning (2007 report)
- perform antiseptic oral care (CDC/HICPAC A-I)
Catheter-associated urinary tract infection
- reduction in unnecessary catheter use (2007 report)
- adherence to aseptic catheter insertion and catheter care (2007 report)
- maintain a closed drainage system and maintain unobstructed urine flow (CDC/HICPAC IB); do not disconnect unless irrigation needed (SHEA/IDSA A-I)
CDC/HICPAC system for rating recommendations:
Category IA. Strongly recommended for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies.
Category IB. Strongly recommended for implementation and supported by certain experimental, clinical, or epidemiologic studies and a strong theoretical rationale.
Category IC. Required for implementation, as mandated by federal or state regulation or standard.
Category II. Suggested for implementation and supported by suggestive clinical or epidemiologic studies or a theoretical rationale.
No recommendation. Unresolved issue. Practices for which insufficient evidence or no consensus regarding efficacy exist.
SHEA/IDSA system for rating recommendations:
Strength of recommendation:
A good evidence to support recommendation
B moderate evidence to support recommendation
C poor evidence to support recommendation
Quality of evidence:
I evidence from > 1 properly randomized controlled trial
II evidence from > 1 well-designed clinical trial w/out randomization; cohort or case-control analytic studies (preferably from > 1 center); multiple time series; or dramatic results from uncontrolled experiments
III evidence from opinions of respected authorities, based on clinical experience, or expert committees
Appendix 2 Sources
- Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep 2002 Oct 25;51(RR-16):1-45. PMID: 12418624.
- Gould CV, Umscheid CA, Agarwal RK, et al, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Atlanta, GA: Centers for Disease Control and Prevention; 2009. Available at: http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed July 29, 2011.
- Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol 2008 Oct;29 Suppl 1:S51-61. PMID: 18840089.
- Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol 2008 Oct;29 Suppl 1:S22-30. PMID: 18840085. Erratum in: Infect Control Hosp Epidemiol 2009 Aug;30(8):815.
- Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol 2008 Oct;29 Suppl 1:S31-40. PMID: 18840087.
- Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol 2008 Oct;29 Suppl 1:S41-50. PMID: 18840088.
- Ranji SR, Shetty K, Posley KA, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Volume 6—Prevention of Healthcare–Associated Infections. Evidence Report/Technology Assessment No. 9 (Prepared by Stanford University–UCSF Evidence-based Practice Center under Contract No. 290-02-0017). Rockville, MD: Agency for Healthcare Research and Quality; January 2007. AHRQ Publication No. 04(07)-0051-6. Available AT: http://www.ncbi.nlm.nih.gov/books/NBK43982/pdf/TOC.pdf. Accessed July 28, 2011.
- Mangram AJ, Horan TC, Pearson ML, et al, and the Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol 1999 Apr;20(4):520-78. PMID: 10219875.
- O’Grady NP, Alexander M, Dellinger EP, et al, for the Centers for Disease Control and Prevention. Guidelines for the prevention of intravascular catheter-related infections. MMWR Recomm Rep 2002 Aug 9;51(RR-10);1-29. PMID: 12233868.
- Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004 Mar 26;53(RR-3);1-36. PMID: 15048056.
- O’Grady NP, Alexander M, Burns LA, et al, and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for the Prevention of Intravascular Catheter-Related Infections, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2011. http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf. Accessed July 29, 2011.
Appendix 3. Recommended Elements for Reporting on Patient Safety Practices
(Source: Shekelle et al. 2010)
The RAND report (Shekelle et al. 2010) specifies that the following elements should be included in reporting on the implementation of PSPs:
- An explicit description of the theory for the chosen intervention components, and/or an explicit logic model for “why this PSP should work.”
- A description of the PSP in sufficient detail that it can be replicated, including the expected change in staff roles.
- Measurement of contexts in the following four domains:
- Structural organizational characteristics (such as size, location, financial status, existing quality and safety infrastructure)
- External factors (such as regulatory requirements or incentive systems)
- Patient safety culture, teamwork, and leadership at the level of the unit
- Availability of implementation and management tools (such as staff education and training, use of internal audit-and-feedback, presence of internal or external individuals responsible for implementation)
- Details of the implementation process, what the actual effects were on staff roles, and how the implementation or the intervention changed over time.
- Assessment of the impact of the PSP on outcomes and possible unexpected effects. Including data on costs, where available, is desirable.
- For studies with multiple interventions, an assessment of the influence of context on intervention and implementation effectiveness (processes and clinical outcomes).
Appendix 4. Literature Search Strategy
(Source: Ranji et al.16)
#1
targets QI strategies that tend to be multi-factorial using relevant MeSH terms and title words
Patient-Centered Care [mh] or Progressive Patient Care [mh] or Critical Pathways [mh] or Delivery of Health Care, Integrated [mh] or Patient Care Team [mh] or Behavior
Control [mh] or ((coordination [tw] or coordinated [tw] or Multifactorial [tw] or Multi-factorial [tw] or Multicomponent [tw] or Multi-component [tw] or multidisciplinary [tw] or multi-disciplinary [tw] or interdisciplinary [tw] or interdisciplinary [tw] or integrated [tw] or community-based [tw] or organized [tw] or comprehensive [tw]) and program*[tw] or care [tw] or approach [tw] or intervention [tw] or strategy[tw] or strategies [tw] or management [tw] or managing [tw] or center*[tw])) or Organization and Administration [mh] or bundle*[tw]
#2
targets TQM and CQI
Total Quality Management [mh] OR Quality control [mh] OR TQM [tw] OR CQI [tw] OR (quality [tw] AND (continuous [tw] OR total [tw]) AND (management [tw] OR improvement [tw]))
#3
targets provider education
Education, Continuing [mh] OR Education, Nursing [mh] OR Education, Medical [mh] OR Inservice Training [mh] OR Programmed Instruction [mh] OR ((Education [tw]AND
Continuing [tw]) AND (medical [tw] OR professional* [tw] OR nursing [tw] OR physician* [tw] OR nurse* [tw])) OR (outreach [tw] AND (visit* [tw] OR educational [tw]) OR (academic [tw] AND detailing [tw]))
#4
targets diffusion of innovation
Diffusion of Innovation [mh] OR (Diffusion [ti] AND (Innovation [ti] OR technology [ti]))
#5
targets audit & feedback, reminder systems, and financial incentives
Medical audit [mh] OR ((Audit [tw] OR feedback [tw] OR compliance [tw] OR adherence [tw] OR training [tw]) AND (improvement* [tw] OR improving [tw] OR improves [tw] OR improve [tw] OR guideline* [tw] OR practice* [tw] OR medical [tw] OR provider* [tw] OR physician* [tw] OR nurse* [tw] OR clinician* [tw] OR academic [tw] OR visit* [tw])) OR Reminder Systems [mh] OR Reminder* [tw] OR ((financial [tw] OR economic [tw] OR physician* [tw] OR patient*) AND incentive* [tw]) OR Reimbursement Mechanisms [mh] or Guideline Adherence [mh] OR practice guidelines [mh]
#6
Medical Informatics [mh] OR computer [tw] OR (decision [tw] AND (support [tw] or analysis [tw))
#7
All QI studies
#1 or #2 or #3 Or #4 or #5 or #6
#8
Surgical site infection terms
Surgical wound infection[mh] OR surgical site infection*[tiab] OR postoperative infection*[ti] OR postsurgical infection*[ti] OR wound infection*[ti] OR sternal
wound infection*[tiab] OR postoperative[ti] OR postsurgical[ti]
#9
Combination of QI terms with SSI terms
#7 AND #8
#10
RCT search
Randomised [ti] OR Randomized [ti] OR Controlled [ti] OR intervention [ti] OR evaluation [ti] OR impact [ti] OR effectiveness [ti] OR Evaluation [ti] OR Studies [ti] OR study [ti] Comparative [ti] OR Feasibility [ti] OR Program [ti] OR Design [ti] OR Clinical Trial [pt] OR Randomized Controlled Trial [pt] OR Epidemiologic Studies [mh] OR Evaluation Studies [mh] OR Comparative Study [mh] OR Feasibility Studies [mh] OR Intervention Studies [mh] OR Program Evaluation [mh] OR Epidemiologic Research Design [mh]
#11
Meta-analysis, systematic review search
((meta-analysis [pt] OR meta-analysis [tw] OR metanalysis [tw]) OR ((review [pt] OR guideline [pt] OR consensus [ti] OR guideline* [ti] OR literature [ti] OR overview [ti] OR review [ti] OR Decision Support Techniques [mh]) AND ((Cochrane [tw] OR Medline [tw] OR CINAHL [tw] OR (National [tw] AND Library [tw])) OR (handsearch* [tw] OR search* [tw] OR searching [tw]) AND (hand [tw] OR manual [tw] OR electronic [tw] OR bibliographi* [tw] OR database* OR (Cochrane [tw] OR Medline [tw] OR CINAHL [tw] OR (National [tw] AND Library [tw]))))) OR ((synthesis [ti] OR overview [ti] OR review [ti] OR survey [ti]) AND (systematic [ti] OR critical [ti] OR methodologic [ti] OR quantitative [ti] OR qualitative [ti] OR literature [ti] OR evidence [ti] OR
evidence-based [ti]))) BUTNOT (editorial [pt] OR comment [pt] OR letter [pt])
#12
All original research
#10 OR #11
#13
Combination of QI terms with SSI terms, limited to original research only
#9 AND #12
#14
#SSI/QI search limited to English only
#13 AND Limits: English
#15
CLABSI search (restrict to English only)
(Catheterization, Central Venous [MeSH] OR central line*[ti] OR central venous catheter*[ti]) AND (Cross infection [mh] OR bacteremia [mh] OR nosocomial [tiab] OR “healthcare associated”[tiab] OR “hospital acquired”[tiab] OR bundle[tiab])
#16
VAP search (restrict to English only)
(Respiration, Artificial[mh] OR mechanically ventilated*[ti] OR intubated*[ti] OR mechanical ventilation*[ti] or ventilator associated*[ti]) AND (Cross infection [mh] OR bacteremia [mh] OR nosocomial [tiab] OR “healthcare associated”[tiab] OR “hospital acquired”[tiab] OR bundle[tiab] )
#17
UCUTI search (restrict to English only)
(Urinary catheterization[mh] OR urinary catheter*[tiab]) AND (Cross infection [mh] OR bacteremia [mh] OR nosocomial [tiab] OR “hospital-acquired”[tiab] OR healthcareassociated”[tiab] OR bundle[tiab])
Supplemental searches
#S1
Nosocomial infection systematic reviews (limited to English only)
Cross infection[mh] AND systematic[sb]
#S2
Handwashing systematic reviews (limited to English only)
Handwashing[mh] AND systematic[sb]
#S3
Author searches
Pronovost p[au] OR Gastmeier P[au] OR Gyssens IC[au]
Appendix 5. Data Abstraction Elements
- Study design
- Context
- Structural organizational characteristics (such as size, location, financial status, existing quality and safety infrastructure)
- Healthcare setting (tertiary care or university hospital, community hospital with residents, non-teaching community hospital, more than one hospital of different types, nursing home, outpatient surgery center, free-standing dialysis center, rehabilitation center, other)
- Clinical setting (intensive care unit, operating room, general inpatient ward other)
- External factors (such as regulatory requirements or incentive systems)
- Patient safety culture, teamwork, and leadership at the level of the unit
- Availability of implementation and management tools (such as staff education and training, use of internal audit-and-feedback, presence of internal or external individuals responsible for implementation)
- Structural organizational characteristics (such as size, location, financial status, existing quality and safety infrastructure)
- QI Strategy
- Target (all clinical staff, physicians, nurses, respiratory therapists, other ancillary staff, patients, other)
- Method of allocation into intervention and control groups
- Type of strategy (see Appendix 1)
- Implementation context (Shekelle et al. 2010)
- Who are the interveners: how were they selected, what role do they play in the organization, what is their relationship to the targets of the intervention?
- Who are the targets of the intervention: how were they selected, what role do they play in the organization, what is their relationship to the patients?
- what specifically are the interveners doing to the intervenees, how consistent is the interveners’ behavior across intervenees?
- what if any new technologies or changes to the physical plant or organizational structures, or policies and procedures were introduced?
- how is the intervention expected to influence the behavior of the intervenee?
- Preventive intervention (see Appendix 2 for list of those to be included)
- Outcome measures
- Preintervention and postintervention infection rates
- Unanticipated complications
- Infection-related complications, mortality
- Preintervention and postintervention adherence to preventive interventions
- Costs, cost-effectiveness, return on investment
Appendix 6. Criteria for Assigning Strength of Evidence
(Source: Shekelle et al.20)




