Background and Objectives for the Systematic Review
Overview
Restless legs syndrome (RLS) or Willis-Ekbom disease is a neurological disorder that causes unpleasant or painful sensations within the legs and a distressing, irresistible urge to move the legs.1 RLS symptoms worsen during inactivity and at night. Partial or complete relief may result from movement such as walking, stretching, or bending of the legs. Such relief is temporary, however, and symptoms return when movement ceases. If the disease progresses, symptoms may occur earlier in the day and intensify even further at night and/or extend beyond the legs to the arms or trunk.2 The clinical course of RLS varies; periods of remission are common, particularly in younger patients and those with milder disease. Severe restless legs syndrome, however, is a chronic progressive disorder that may require long-term treatment.2
Prevalence estimates for RLS range from 3 to 10 percent,3 and are higher for women and older people.3,4 Different approaches to diagnosing RLS and defining its severity lead to the large variance in prevalence estimates, as does the fact that many RLS questionnaires do not account for individuals with other conditions with similar symptoms (e.g. neuropathies, pain syndromes).5
RLS is believed to be idiopathic or primary RLS, or secondary to other conditions such as iron deficiency, end-stage renal disease and pregnancy6,7. Secondary RLS often starts later in life, is associated with more rapid progression than idiopathic RLS, and is often resolved when the underlying condition is treated.8,9
RLS has a wide spectrum of disease severity.2 Patients with mild RLS may experience its symptoms as only a minor annoyance. However, severe RLS can have a crippling impact on quality of life. It can prevent participation in occupational or social activities, and reduce function and emotional well-being. RLS-induced sleep disruption may lead to poor daytime functioning, anxiety, and depression. Indeed, sleep deprivation and daytime fatigue are the most common reasons RLS patients seek treatment.10
Treatments for RLS include nonpharmacological and pharmacological options (Table 1). Pharmacological treatment is generally reserved for patients with severe RLS.11 The major classes of drugs used are dopaminergic agents, sedative hypnotic agents, anticonvulsive agents, opiates, and iron.12 Long-term treatment with dopaminergic agents can lead to a paradoxical worsening of symptoms known as augmentation, which is a significant complication.13 The primary goal of RLS treatment is to manage symptoms and improve patient function and quality of life. Except for the limitations on pharmacological therapy imposed by pregnancy,14 and the use of iron replacement for those with iron deficiency, treatment options are unlikely to vary for primary and secondary forms of RLS.15 For patients with secondary RLS, the recommendation is to treat the associated condition first, whenever it is possible to do so. Clinical experience suggests that RLS associated with pregnancy is resolved postpartum in most of the patients;16 however, there have been no evaluations of therapy in this population and very little is known about women with pregnancy-induced RLS whose symptoms persist even after delivery.7
| Pharmacological Treatments | ||||
|---|---|---|---|---|
| Class | Generic Name | US Trade Name | FDA Approved for RLS? | Adverse Effects+ |
| Dopaminergic agents | Levodopa | Sinemet® | Nausea or vomiting;
orthostatic hypertension; hallucination; augmentation of symptoms; insomnia; nasal congestion and fluid retention; and impulse control disorders. |
|
|
Ropinirole |
Requip® |
Yes |
||
|
Pramipexole |
Mirapex® |
Yes |
||
|
Ritigotine |
Neupro® |
|||
| Sedative-hypnotics |
Clonazepam |
Rivotril® |
Tolerance; sedation; gait instability, and impotence. |
|
|
Temazepam |
Restoril® |
|||
|
Oxazepam |
Serax® |
|||
| Anticonvulsants |
Gabapentin Enacarpil |
Horizant® |
Yes |
Dizziness; fatigue; somnolence; ataxia. |
|
Gabapentin |
Neurontin® |
|||
|
Pregabalin |
Lyrica® |
|||
| Opioids |
Hydrocodone |
-Vicodin® -Lortab® |
Sedation; pruritus; constipation; nausea or vomiting; dry mouth; dependence; exacerbation of sleep apnea. |
|
|
Codeine |
Tylenol # 3 w/codeine® |
|||
|
Tramadol |
-Ultram®
|
|||
|
Oxycodone or oxycodone-XR |
-Tylox®
|
|||
|
Methadone |
-Methadose®
|
|||
|
Morphine Sulphate-XR |
Depodur® |
|||
|
Iron |
Constipation; nausea; reflux; abdominal pain; diarrhea. |
|||
| Non-pharmacological Treatments | ||||
|
||||
| †Adverse effects of treatments are specific to use of these drugs for RLS and were derived primarily from Earley10 with additional input from clinical experts. | ||||
- Controversy and Uncertainty
Clinicians face substantial uncertainty related to defining RLS, assessing disease severity, and evaluating the risk/benefits of treatment. While these challenges are common to both primary care and specialty settings, they may be more pronounced in primary care. Specific issues that affect clinical practice include:
- RLS Diagnosis: primary care versus specialty practice
RLS is diagnosed based on clinical history using standard criteria developed by the International Restless Legs Study (IRLS) group in 199517 and revised in a consensus conference at the NIH in 2003.9 The use of standard criteria is common in clinical research and possibly in specialty practice. However, in primary care, the standard criteria may be less consistently applied. As a result, patients may be misdiagnosed, misclassified, and receive unnecessary or ineffective treatment. Direct-to-consumer advertising may also result in patients requesting potentially inappropriate pharmacological treatments for RLS-like symptoms. - Distinguishing RLS from other disorders
Reliable diagnosis and treatment of RLS requires distinguishing it from disorders that present similar symptoms.18 “Mimic” conditions sometimes satisfy the standard RLS criteria, and thus must be ruled out via neurological examination in cases of clinical uncertainty.18
Many patients with RLS also experience semi-rhythmic limb movements called periodic limb movements (PLM) during wakefulness or sleep. However, these movements are not specific to RLS;19 they may also occur among older adults, in those taking antidepressants, and as a result of certain neurological and sleep disorders (e.g. narcolepsy).20 Therefore, RLS is distinct from sleep disorders such as periodic limb movements disorder (PLMD). - Assessing risk/benefits of treatment
RLS encompasses a broad range of condition severity.9 Pharmacologic treatment is necessary only for those for whom the disease significantly impacts quality of life.21,22 For individuals with mild or moderate symptoms, the risks/benefits of therapy are unclear. In addition, long-term risks and benefits of treatment are unclear for children and older adults with multiple comorbidities. - Measuring changes in disease status and impact of treatment
Lack of objective measures for assessing disease status presents a challenge in clinical practice.23 Typically, clinical interviews are used to assess disease severity and treatment-induced changes in disease status. In research settings, the same assessments are made using specific rating scales such as the International Restless Legs Study Group (IRLS) scale and Clinical Global Impressions (CGI) scale.23 However, the results of RLS severity scales cannot be meaningfully interpreted in the absence of clearly defined “minimum clinically important differences” (MCIDs). MCIDs are the smallest increments of improvement considered worthwhile by the patient. Without established MCIDs, we cannot be certain that a change in scale score reflects improvements that patients consider significant. - Durability and sustainability of treatment benefits
Durability of treatment benefits and sustainability of treatments over time are critical issues. Many patients with RLS report switching between treatments or drug classes due to treatment side effects, or because the treatment benefits are not sustained. - Long-term benefits and harms of treatment:
Substantial uncertainty exists about the long-term benefits and harms of treatments for RLS. Most of what we know about the effectiveness of pharmacological treatments for RLS comes from short-term clinical trials. Yet, the disease is chronic, often requiring life-long treatment. Furthermore, augmentation, a treatment-induced exacerbation of symptoms in response to dopaminergic therapy, can occur during the first 2 years of treatment and sometimes many years into treatment.24
Several developed scales are used to assess RLS severity, impact, and specific health outcomes. (Table 2).23 The International Restless Leg Study group (IRLS) scale is most widely reported. MCIDs have not been defined for these scales.23
| Severity and Impact of Disease | ||
|---|---|---|
| Scale | Objective/Description | Components of the Scale |
| International RLS Study group scale (IRLS) |
|
|
| Clinical Global Impressions (CGI)[1] |
|
|
| Impact of RLS on Quality of Life | ||
| Scale | Objective/Description | Components of the Scale |
| Restless Legs Quality of Life
Instrument (RLS-QLI) |
|
|
| Hopkins RLS Quality of Life
Questionnaire (RLSQoL) |
|
|
| RLS Quality of Life Questionnaire
(Qol-RLS) |
|
|
| Impact of RLS on Sleep | ||
| Scale | Objective/Description | Components of the Scale |
| Epworth Sleepiness Scale |
|
|
| Medical Outcomes Study Sleep Scale |
|
|
| Pittsburgh Sleep Quality Index |
|
|
| Augmentation | ||
| Scale | Objective/Description | Components of the Scale |
| Augmentation* Severity Rating Scale
(ASRS) |
|
|
|
*Augmentation is characterized by: greater intensity of symptoms; earlier onset of symptoms; shorter latency to onset of symptoms during inactivity; and spread of symptoms to other body parts (usually the arms, but also to the trunk and the face) |
||
Relevance of the Review to Patients, Practitioners and to the Research Community
We will systematically review the literature to assess the benefits and harms of treatment, especially long-term outcomes. We will evaluate methods used to define RLS, assess its severity, and measure treatment benefits and harms. Further, we will identify gaps in the available evidence and develop a future research agenda.
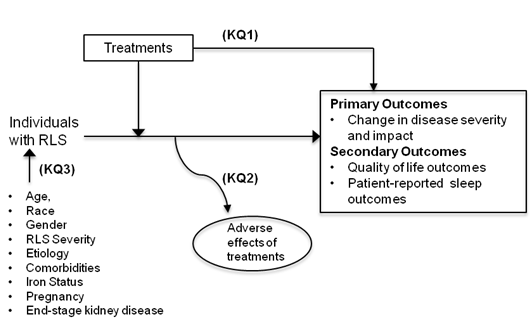
RLS treatment choices vary by patient age and by the severity and impact of the disease.11 For patients whose symptoms are mild (and/or episodic or intermittent), the critical issue is how to evaluate need for treatment based on the degree to which symptoms affect the patient’s quality of life. For patients suffering from severe RLS, the critical issue is how to identify the treatment options with the greatest long-term benefits and the least harms. Treating children and older adults with RLS presents specific challenges. We do not know the impact of long-term use of these drugs in children. Neither do we know the risks/benefits of the drugs for older adults who take several medications for multimorbidities.
The Key Questions
We developed the key questions after a topic refinement process that included a preliminary review of the literature and consultation with a key informant panel of RLS experts and stakeholders. Key informants identified specific salient issues, including the complexity of determining need for treatment, and uncertain long-term risks/benefits of treatment. Additionally, the panel emphasized the need to examine treatment durability and sustainability, because patients using RLS medications long-term often report the need to switch treatments as benefits diminish or cease over time. Based on key informant input, we made the following changes.
- Expanded the population to include all individuals diagnosed with RLS.
- Added specific questions to address durability and sustainability of treatments and the long-term harms of treatment.
The draft key questions were posted for public comment on the AHRQ Effective Health Care Program website for additional feedback from August 2, 2011 to August 30, 2011. We also sought input from a technical panel of experts (TEP) convened to provide methodological and content expertise to the review. In response to public comments and input from the TEP, we revised the key questions, adding iron status to the list of patient characteristics that may affect outcomes. The TEP endorsed restricting study scope to individuals diagnosed with RLS and excluding studies on periodic limb movement disorder or other sleep-related conditions. The TEP also helped us prioritize outcomes on the basis of their relevance to improvements in patient function and quality of life. Informed by input from the TEP, we chose the International RLS (IRLS) scale score as the primary outcome and Clinical Global Impression (CGI) scale score, disease-specific quality of life scale score, and patient-reported sleep quality as secondary outcomes. We expanded the list of nonpharmacological treatments to include counter pulsation devices and compression stockings.
The final key questions are:
Question 1
What is the comparative effectiveness of treatments for restless legs syndrome (RLS)?
- What are the benefits from RLS treatments when compared to placebo or no treatment?
- What are the benefits from RLS treatments when compared to other active treatments?
- What is the durability and sustainability of treatment benefits?
Question 2
What are the harms from RLS treatments?
- What are the harms from RLS treatments when compared to placebo or no treatment?
- What are the harms from RLS treatments when compared to other active treatments?
- What are the long-term harms from treatment?
Question 3
What is the effect of patient characteristics (age, gender, race, comorbidities, disease severity, etiology, iron status, pregnancy, end-stage renal disease) on the benefits and harms of treatments for RLS?
The definitions of population, intervention/comparator, outcomes, setting and time frame are:
Population
- Individuals with restless legs syndrome
Major subgroup: Older adults (age 65+) with comorbidities
Patient characteristics of interest, which may modify RLS disease course and treatment outcomes, include:- Age
- Race
- Gender
- RLS Severity
- Comorbidities
- Etiology (i.e., primary or secondary RLS)
- Iron Status
- Pregnancy
- End-stage kidney disease
Interventions
- Pharmacological treatments (dopaminergic agents, sedative-hypnotics, anticonvulsants, opioids, and iron supplementation)
- Nonpharmacological treatments (moderate exercise, hot or cold bath, limb massage, sleep hygiene, acupuncture, herbal medicines, cognitive behavioral therapy, counter pulsation devices, compression stockings, eliminating precipitants of RLS)
Interventions may include combination of one of more of pharmacological or non-pharmacological treatments.
Comparators
- Placebo (or sham treatments), no treatment, or other active comparator
Outcomes
Primary Outcomes
- Change in disease severity and impact assessed using International RLS (IRLS) rating scale
Secondary Outcomes
- Change in Clinical Global Impression (CGI) scale score
- Change in quality of life as measured by disease-specific scale (e.g., Restless legs quality of life instrument, Hopkins RLS quality of life questionnaire, RLS quality of life questionnaire)
- Change in patient-reported sleep outcomes measured using a validated sleep scale (e.g., Epworth sleepiness scale, Medical Outcomes Study sleep scale, Pittsburgh Sleep Quality Index)
Harms of treatment
- All reported adverse reactions and effects including Augmentation (Harms specific to each class of drugs are listed in Table 1)
- Treatment discontinuation due to adverse effects (number of patients experiencing adverse events, number of drop-outs due to adverse events)
For each of the outcomes, we will analyze total scale scores from validated scales noted above. For each scale, we will try to determine, the minimum change in score that translates to clinically meaningful improvement. To analyze clinically meaningful response to treatment we will set responder criteria on these scale scores to be: 1) resolution of symptoms (IRLS scale score=0); 2) percent of patients with reduction of symptoms from very severe or severe to mild (IRLS<10); 3) More than 50% change in IRLS score from baseline; or 4) percent of patients who are much improved or very much improved on the CGI scale.
Timing
- Minimum of 4 weeks. (short-term: < 6 months; intermediate 6-24 months; long term > 24 months)
Setting
- Outpatient settings
a Durability refers to treatment benefits that hold up over time while sustainability refers to tolerability of treatments over time.
Analytic Framework
Methods
A. Criteria for Inclusion/Exclusion of Studies in the Review
Below we describe the general criteria used to identify eligible randomized controlled trials (RCTs) and observational studies. We will use evidence from observational studies to assess long-term harms of treatment; of particular interest are long-term, open label trials and followup studies.
Inclusion Criteria for RCTs:
- Randomized controlled trials (parallel-group as well as crossover) of pharmacological and nonpharmacological interventions for RLS.
- Studies involving individuals of all ages diagnosed with RLS (according to standard IRLS criteria or other equivalent clinical criteria). We will stratify patients by age (<18 yrs, 18-65, > 65 yrs).
- Studies involving participants from primary or specialty care settings.
- Studies that include individuals with RLS comorbid with iron deficiency, pregnancy, or end-stage renal disease.
- All pharmacological or nonpharmacological treatments for RLS compared to other active treatment, sham treatment, or a placebo.
- Studies that record at least one outcome as change in one of the primary or secondary outcomes as specified in Table 2.
- Study duration is at least 4 weeks.
Exclusion Criteria for RCTs:
- Non-English language studies.
Rationale: Our review of the literature and discussions with the technical expert panel indicate that studies relevant to this review, including clinical trials from countries in Europe are published in English language. Therefore, restricting studies to those published in English would not affect the findings of the review.
- Studies that report periodic limb movements disorder (PLMD) or other sleep-related conditions that are not RLS.
- Studies that report only polysomnographic or other objective sleep-related measures.
- Studies that evaluate only acute effects (< 4 weeks) of treatment.
Inclusion/Exclusion criteria for observational studies (for Key Question 2):
- Studies that evaluate adverse effects of pharmacological and nonpharmacological treatments in individuals diagnosed with RLS will be included. Long-term, open-label studies or followup studies of treatments for RLS are of particular interest and will be included. To evaluate harms of interest, we will include evidence from case reports or case series. We will exclude observational studies that do not evaluate harms from treatments for RLS.
B. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies to Answer the Key Questions
We will search MEDLINE (via PubMed), EMBASE, the Cochrane Library and the Cochrane Central Trials Registry, and Natural Standards. We will use the EMBASE database to retrieve studies published in European journals that may not be indexed in MEDLINE. The preliminary search strategy for MEDLINE is listed in Appendix A. We will adapt this search strategy to conform to the syntax requirements of individual bibliographic databases. We will also evaluate the bibliographies of included primary studies and any identified systematic or nonsystematic reviews.
We will search the grey literature sources such as ClinicalTrials.gov, the International Controlled Trials Registry Platform (ICTRP), and the NIH RePORTer to identify completed clinical trials and to check for publication bias.
Two independent reviewers will screen titles/abstracts using the inclusion/exclusion criteria listed above (pg.10). Articles included by either reviewer will undergo full-text screening, after which two reviewers must agree on a final inclusion/exclusion decision. Disagreements will be resolved by discussion or, when needed, by consultation with a third reviewer. Articles meeting eligibility criteria will be included for data abstraction.
After the draft report is submitted, we will follow the same procedure to update the literature search covering the interval since completion of the original search.
C. Data Abstraction and Data Management
We will download results from screening into EndNote® reference-management system. Data from individual studies will be abstracted directly into evidence tables by one reviewer and validated by a second reviewer. Disagreements will be resolved by discussion or, when needed, by consultation with a third reviewer. We will abstract data on study design; inclusion/exclusion criteria used to select study participants; patient characteristics (age, gender, race, disease severity, comorbidities, iron status); methods used to define RLS and assess severity of disease; and definition of clinically significant change in disease status used in individual studies. Data elements will include descriptors to assess intervention/exposure details; outcomes; methodological quality; and study applicability.
D. Assessment of Methodological Quality of Individual Studies
The primary and secondary abstractor/evaluator will independently assess the risk of bias of each eligible study using tools specific to study design. Disagreements will be resolved between the two reviewers by discussion or, when needed, by consultation with a third reviewer.
Blinding is a key component of assessing overall quality because assessment of treatment effectiveness is based primarily on patient reported outcomes and treatments are associated with a high placebo rate. For each RCT, we will assess risk of bias using the Cochrane risk of bias tool. We will evaluate random allocation of the subjects to the treatment groups; adequacy of allocation concealment and randomization; masking of the treatment status; intention-to-treat principles; and selective outcome reporting. We will assume a low risk of bias when RCTs meet all the quality criteria; a moderate risk of bias if at least one of the quality criteria was not met; and a high risk of bias if two or more quality criteria were not met. We will conclude there is an unknown risk of bias for the studies with poorly reported quality criteria.
For observational studies, we will evaluate strategies used to reduce selection bias; adjustments made for confounding; validity of outcome measures; and length and completeness of follow-up. We will use the RTI-item bank for items specific to these quality elements.25
E. Data Synthesis
We will summarize the primary literature by abstracting relevant continuous and categorical data. We will determine the feasibility of completing a quantitative synthesis (i.e., meta-analysis). Feasibility depends on the volume of relevant literature, conceptual homogeneity of the studies, and completeness of the results reporting. We anticipate that studies may report dichotomous outcomes (e.g., global assessment of improvement) and continuous outcomes (e.g., change in IRLS score). We will use the weighted mean difference when studies use the same outcome scale; otherwise standardized mean differences will be used. Data will be pooled using random-effects model. We will assess heterogeneity in results with Chi-square and I-square tests.
Data synthesis will be stratified by patient characteristics (age, comorbidities, disease severity, iron status). We will attempt to derive a metric to assess clinically meaningful improvements in symptom frequency and severity by determining the number and percentage of patients who have had remission of RLS symptoms and who adhere to treatment. We will assess the number and percentage of patients that report adverse effects, especially augmentation. Where possible, we will stratify results by followup duration (short, intermediate, and long-term). Pre-planned sensitivity analyses include: age, disease severity, iron status, and comorbidities.
F. Grading the Evidence for Each Key Question
We will assess the strength of evidence for each key question using the approach described in the EPC methods manual. In brief, the EPC approach requires assessment of four domains for each outcome:
- Risk of bias (internal validity)
- Consistency (similarity of effect sizes of included studies)
- Directness (single direct link between intervention and outcome)
- Precision (degree of certainty surrounding an effect estimate)
Additional domains are to be used when appropriate, including coherence, dose-response association, impact of plausible residual confounders, strength of association (magnitude of effect), and publication bias. These domains will be considered qualitatively and a summary rating will be assigned after discussion by two reviewers as “high,” “moderate,” or “low” strength of evidence. We will assign a summary rating of “insufficient” when evidence is unavailable. These ratings will be interpreted as follows:
- High: High confidence that the evidence reflects the true effect; further research is very unlikely to change the confidence in the estimate of effect.
- Moderate: Moderate confidence that the evidence reflects the true effect; further research may change our confidence in the estimate of effect and may change the estimate.
- Low: Low confidence that the evidence reflects the true effect; further research is likely to change the confidence in the estimate of effect and is likely to change the estimate.
- Insufficient: Evidence either is unavailable or does not permit a conclusion.
G. Assessing Applicability
To access applicability of individual studies, we will evaluate the eligibility requirements used to select patient population, baseline disease severity, and length of followup. The length of followup is important to establish long-term benefits and harms of treatment and will be a key variable in assessing applicability. We will describe the enrolled population and make qualitative comparisons to population-based estimates of disease prevalence and severity.
References
- Ekbom K. Restless legs syndrome. Neurology. 1960;10:868–873.
- Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nature Reviews Neurology. 2010;6(6):337–346.
- García-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: The current status. Sleep Med Rev. 2006;10(3):153–167.
- Allen RP. Restless Legs Syndrome Prevalence and Impact: REST General Population Study. Archives of Internal Medicine. 2005;165(11):1286–1292.
- Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”). Sleep medicine. 2009;10(9):976–981.
- Manconi M, Govoni V, De Vito A, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep medicine. 2004;5(3):305–308.
- Cesnik E, Casetta I, Turri M, et al. Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology. 2010;75(23):2117–2120.
- Allen R, Earley C. Defining the phenotype of the restless legs syndrome (RLS) using age-of-symptom-onset. Sleep medicine. 2000;1(1):11–19.
- Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. In: Sleep medicine.Vol 4. 2003:101–119.
- Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26(6):925–935.
- Silber MH, Ehrenberg BL, Allen RP, et al. An algorithm for the management of restless legs syndrome. Mayo Clin. Proc. 2004;79(7):916–922.
- Earley CJ. Restless Legs Syndrome. N Engl J Med. 2003;348(21):2103–2109.
- García-Borreguero D, Allen RP, Benes H, et al. Augmentation as a treatment complication of restless legs syndrome: concept and management. Mov Disord. 2007;22 Suppl 18:S476–84.
- Djokanovic N, Garcia-Bournissen F, Koren G. Medications for restless legs syndrome in pregnancy. J Obstet Gynaecol Can. 2008;30(6):505–507.
- Allen RP. Controversies and Challenges in Defining the Etiology and Pathophysiology of Restless Legs Syndrome. The American Journal of Medicine. 2007;120(1):S13–S21.
- Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065–1069.
- Walters AS, Walters AS, Aldrich MS, et al. Toward a better definition of the restless legs syndrome. Mov Disord. 1995;10(5):634–642.
- Benes H, Walters AS, Allen RP, Hening WA, Kohnen R. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(S18):S401–S408.
- Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: Prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10(3):169–177.
- Winkelman JW. Periodic Limb Movements in Sleep — Endophenotype for Restless Legs Syndrome? N Engl J Med. 2007;357(7):703–705.
- Silber MH, Ehrenberg BL, Allen RP, et al. An algorithm for the management of restless legs syndrome. Mayo Clin. Proc. 2004;79(7):916–922.
- García-Borreguero D, Stillman P, Benes H, et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011;11(1):28.
- Kohnen R, Allen RP, Benes H, et al. Assessment of restless legs syndrome—Methodological approaches for use in practice and clinical trials. Mov Disord. 2007;22(S18):S485–S494.
- Silver N, Allen RP, Senerth J, Earley CJ. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep medicine. 2011;12(5):440–444.
- Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2011.
Definition of Terms
| RLS Diagnostic | Essential Criteria for diagnosis of RLS |
|---|---|
| Criteria |
|
| Augmentation |
Treatment induced worsening of symptoms in RLS patients being treated with dopaminergic agents. (e.g.,earlier onset of symptoms at night, shorter latency to onset of symptoms, appearance of daytime symptoms, and spread of symptoms to other body parts) |
| PLMD |
Periodic limb movement disorder. RLS patients may experience semi-rhythmic movements of the legs called periodic limb movements (PLM) during wakefulness or sleep. However, PLM are not specific to RLS and PLMD is a distinct sleep disorder. |
Summary of Protocol Amendments
In the event of protocol amendments, the date of each amendment will be accompanied by a description of the change and the rationale.
Review of Key Questions
For all EPC reviews, key questions were reviewed and refined as needed by the EPC with input from Key Informants and the Technical Expert Panel (TEP) to assure that the questions are specific and explicit about what information is being reviewed. In addition, for Comparative Effectiveness reviews, the key questions were posted for public comment and finalized by the EPC after review of the comments.
Key Informants
Key Informants are the end users of research, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making health care decisions. Within the EPC program, the Key Informant role is to provide input into identifying the Key Questions for research that will inform healthcare decisions. The EPC solicits input from Key Informants when developing questions for systematic review or when identifying high priority research gaps and needed new research. Key Informants are not involved in analyzing the evidence or writing the report and have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism.
Key Informants must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their role as end-users, individuals are invited to serve as Key Informants and those who present with potential conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Technical Experts
Technical Experts comprise a multi-disciplinary group of clinical, content, and methodologic experts who provide input in defining populations, interventions, comparisons, or outcomes as well as identifying particular studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicted opinions are common and perceived as health scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, design and/or methodological approaches do not necessarily represent the views of individual technical and content experts. Technical Experts provide information to the EPC to identify literature search strategies and recommend approaches to specific issues as requested by the EPC. Technical Experts do not do analysis of any kind nor contribute to the writing of the report and have not reviewed the report, except as given the opportunity to do so through the public review mechanism.
Technical Experts must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals are invited to serve as Technical Experts and those who present with potential conflicts may be retained. The TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Peer Reviewers
Peer reviewers are invited to provide written comments on the draft report based on their clinical, content, or methodologic expertise. Peer review comments on the preliminary draft of the report are considered by the EPC in preparation of the final draft of the report. Peer reviewers do not participate in writing or editing of the final report or other products. The synthesis of the scientific literature presented in the final report does not necessarily represent the views of individual reviewers. The dispositions of the peer review comments are documented and will, for CERs and Technical briefs, be published three months after the publication of the Evidence report.
Potential Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Invited Peer Reviewers may not have any financial conflict of interest greater than $10,000. Peer reviewers who disclose potential business or professional conflicts of interest may submit comments on draft reports through the public comment mechanism.
Appendix A
Ovid MEDLINE Search Strategy for Primary Studies: First Iteration
1 "restless leg$ syndrome".mp. (2470)
2 "Ekbom syndrome".mp. (27)
3 Randomized Controlled Trials as Topic/ (76575)
4 randomized controlled trial/ (316968)
5 random allocation/ (72869)
6 double blind method/ (112853)
7 single blind method/ (15532)
8 clinical trial, phase i.pt. (11643)
9 clinical trial, phase ii.pt. (18390)
10 clinical trial, phase iii.pt. (6549)
11 clinical trial, phase iv.pt. (642)
12 controlled clinical trial.pt. (83518)
13 randomized controlled trial.pt. (316968)
14 multicenter study.pt. (136622)
15 clinical trial.pt. (468143)
16 exp Clinical Trials as topic/ (249329)
17 (clinical adj trial$).tw. (161454)
18 ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw. (110255)
19 PLACEBOS/ (30189)
20 placebo$.tw. (132821)
21 randomly allocated.tw. (12928)
22 (allocated adj2 random$).tw. (15197)
23 or/3-22 (982769)
24 or/1-2 (2483)
25 24 and 23 (385)
26 (case reports or comment or editorial or historical article or letter or news or newspaper article or"review").pt. (4441578)
27 25 not 26 (283)
28 Epidemiologic studies/ (5169)
29 exp case control studies/ (526179)
30 exp cohort studies/ (1132983)
31 case control.tw. (57475)
32 (cohort adj (study or studies)).tw. (55811)
33 (Follow up adj (study or studies)).tw. (32483)
34 (observational adj (study or studies)).tw. (27840)
35 Longitudinal.tw. (105418)
36 Retrospective.tw. (200756)
37 cross sectional.tw. (114815)
38 cross-sectional studies/ (131338)
39 or/1-2 (2483)
40 or/28-38 (1509219)
41 39 and 40 (440)
42 (case reports or comment or editorial or historical article or letter or news or newspaper article or "review").pt. (4441578)
43 41 not 42 (382)



