Attention-Deficit/Hyperactivity Disorder (ADHD) is the single most prevalent behavioral and mental health problem in youth. Approximately 10 percent of US children have received a clinical diagnosis of ADHD.1 Clinical diagnoses have increased steadily over time,2 though the higher rates seem attributable to changing clinical practices rather than to an increase in true population rates. The prevalence of ADHD based on rigorous diagnostic procedures is approximately 5.3 percent, a rate that has remained constant over the more than 20 years when diagnostic criteria have not changed and that is similar across geographic regions worldwide.3 This rate, when compared with the much higher rates of clinical diagnoses, suggests that a large number of youth may be receiving a diagnosis when they should not be, though the increasing rates of diagnosis could also represent the clinical recognition of youth who have clinically significant and functionally impairing ADHD symptoms but who may not meet full, formal diagnostic criteria.4 ADHD is more than twice as likely to be diagnosed in boys than in girls.1 It is a more prevalent diagnosis in youth from low-income families5 and in Caucasian compared to Black, Hispanic, and Asian youth,6 though diagnostic bias and cultural influences may contribute to these socioeconomic, ethnic, and racial disparities in diagnostic rates.7, 8
Diagnosis of ADHD
The first question patients, parents, teachers, and clinicians ask when considering ADHD is, “Does this child truly have ADHD?” Unfortunately, clinician judgement, especially by non-specialist clinicians in primary care, is poor in diagnosing ADHD.9 Accurately identifying youth who have ADHD has proved difficult at a population level, in part because diagnoses are often made using subjective clinical impressions and limited diagnostic tools. These tools include structured and semi-structured parent, youth, and teacher questionnaires. They represent an improvement over unsupported clinician judgement, but they are nevertheless highly subjective, prone to disagreement across reporters,10 and yield many false positive diagnoses.11, 12 Again it is possible, and even likely, that many clinical diagnoses are made in youth who have clinically significant and impairing ADHD symptoms but who do not meet full formal diagnostic criteria, since increasing evidence suggests that ADHD symptoms are continuously distributed quantitative traits and therefore lie on a continuum of severity in the general population.13-15 More objective diagnostic tools have been proposed, including activity monitors,16 neuropsychological test measures,17-20 biomarkers such as genotyping,21 electrophysiological indices,22, 23 and MRI measures,24, 25 though their reliability and validity generally have not been assessed rigorously, and they are not yet established diagnostic tools.
It is essential to know how the comparative accuracy of these diagnostic tools varies by clinical setting, including primary care or specialty clinic, and/or patient subgroup, including age, sex, socioeconomic status, racial or ethnic group, comorbid illnesses, or other risk factors associated with ADHD. The accuracy of an ADHD diagnosis is especially poor in preschool-aged children, for whom hyperactivity, general rambunctiousness, and difficulties with impulse control are often relatively normative and difficult to distinguish from ADHD-related behaviors. Preschool youth also typically do not have the same classroom expectations for behavioral self-regulation that children in elementary school are expected to have,26 further obscuring the distinction between ADHD and neurotypical early childhood behaviors.
ADHD diagnosis is normally based on an assessment to determine whether the patient meets the criteria described in the DSM-5.27 Rating scales, which can be completed by parents, teachers, and/or patients, are used to evaluate the frequency and severity of each of the 18 symptoms in DSM-527 (9 symptoms related to inattention, and 9 symptoms related to hyperactivity/impulsivity), as well as the degree of symptom-related impairment across settings (e.g., home, school, work). Rating scale data are integrated with a clinical interview to determine the onset, course, duration, and impairment associated with symptoms. In addition, screening and clinical evaluation of potential comorbid psychiatric conditions is a key part of the diagnostic process. Important questions remain about the accuracy of this approach in primary care settings. A particular challenge is separating ADHD from other conditions that may appear similar (e.g., anxiety, conduct disorders, speech or language delay, other developmental disorders) and determining whether another condition may better explain ADHD symptoms or is present as a comorbid diagnosis.
Inaccurate diagnoses of ADHD can lead either to the administration of treatments, usually stimulant medications, in children who do not need them, or to the withholding of treatment and services for those who would benefit from such treatments.26, 28 Prescription of stimulant medications has doubled in the last decade,29 with a US prevalence in 2019 of approximately 6 percent, and as high as 14 percent regionally.30 These rates are higher than the 5.3 percent population prevalence of rigorously diagnosed ADHD,31 suggesting that many youth may be receiving stimulants when they do not have ADHD.31, 32 These trends have created alarm in the lay public, policy makers, and health care providers.32, 33 Adding to their concern is that diversion and abuse of stimulants is common, particularly in college students.34 Little is known or understood about how the risk for diversion and abuse of stimulant medications approved for ADHD varies with patient characteristics (e.g., as a function of age, race/ethnicity, or socioeconomic status). Conversely, only about half of US children who receive a clinical diagnosis of ADHD are treated with stimulants,35 suggesting a large number of children are not receiving medication when perhaps they should be. Additional important clinical consequences of an incorrect diagnosis include stigmatizing youth unnecessarily with a diagnosis of ADHD26, 36 (i.e., “labeling harms,” which can impair self-esteem or reduce future educational attainment or career opportunities).37-39 Misdiagnosis of ADHD not only leads to its overdiagnosis or underdiagnosis, but it can also can lead to incorrectly diagnosing as ADHD other conditions that share symptoms with ADHD (e.g., anxiety, conduct disorders, speech or language delay, complex trauma, difficult home environments, attachment problems or other medical disorders/diseases or developmental disorders).40-43 Thus, treating disorders misconstrued as ADHD may withhold appropriate psychosocial and psychological therapies for those conditions and instead inappropriately treat them with stimulants and other ADHD therapies that may have little or no effectiveness in treating those conditions.
Treatment of ADHD
Once a diagnosis of ADHD is made, patients and their parents ask, "What treatment should be undertaken?" The answer to this question is challenging for most clinicians and requires a detailed and accurate understanding of the comparative safety and effectiveness of pharmacologic and behavioral treatments for improving not only the immediate symptoms of ADHD, but also the long-term outcomes that ADHD is known to affect, such as academic and occupational success, depression, substance abuse, and conduct or antisocial behaviors.44 This answer, however, is always conditioned on characteristics of the individual child or the child’s environment that are known to modify response to treatment. These "tailoring variables" can include patient age, ADHD subtype (primarily inattentive, hyperactive/impulsive, or combined), socioeconomic status, race and ethnicity, prior trauma history, co-occurring conditions (e.g., depression or anxiety), family conflict, and biomarker status (e.g., genotype, cognitive testing profile).45, 46 Possible benefits of medication must be weighed against risks and side effects. Many parents and clinicians do not have ready access to information that can help them identify and assess these potential risks and whether their child is likely to respond better or worse to any specific possible treatment they might undertake.
Treatment strategies for ADHD are diverse and can be divided into pharmacologic and nonpharmacologic therapies. The frontline treatment for ADHD is stimulant medication, either methylphenidate or amphetamine derivatives, with or without combined psychological and behavioral therapies. The main categories of pharmacologic therapies include stimulants, selective norepinephrine reuptake inhibitors, alpha-2 agonists, and antidepressants. Nonpharmacologic therapies include psychosocial interventions, behavioral interventions, school-based interventions, cognitive training therapies, learning training, biofeedback or neurofeedback, parent behavior training, dietary supplements, elimination diets, vision training, and chiropractic treatment.
In children over the age of 5, the American Academy of Pediatrics (AAP) recommends stimulants as the first line of therapy.18 It is unclear whether there is a significant benefit of combining behavioral therapy with stimulant therapy, or whether nonpharmacologic therapy may be effective. Adverse effects of pharmacologic treatment depend on the specific intervention and may include gastrointestinal symptoms, changes in appetite, and sleep disturbance.47 Treatment can also lead to personality changes or perceived loss of spontaneity. Individuals who are initially misdiagnosed or who have inadequate monitoring may be overtreated with stimulant medications. Overtreatment leads to the risk of treatment with little or no benefit or to unnecessary side effects.
Effects on short-term outcomes for either class of stimulant medication have been large, whereas effects for psychological and behavioral therapies on short-term outcomes generally have been small or moderate in magnitude.47 Long-term outcomes for both medication and non-medication therapies have been less well studied,47 and little is known about which treatment to begin first and for whom, or how best to sequence treatments for ADHD when the first intervention proves ineffective or insufficient. SMART (Sequential Multiple Assignment Randomized Trial) study designs have begun to emerge to help identify the best sequences of treatment and have begun to call into question the dominant practice of beginning treatment with medication rather than behavioral therapy.48 SMART designs also help identify which treatment sequences work best for which type of patient – young or old, in which ethnic group, with which comorbid illnesses, and with which specific genotypes.21, 49-52 Recent advances in the development and testing of novel therapies for ADHD warrant systematic review of their efficacy and effectiveness and will provide information eagerly awaited by clinicians and stakeholders. These novel therapeutics include cognitive training,53-56 game-based digital devices such as the FDA-approved EndeavorRx,57 and neuromodulation techniques58 such as repetitive Transcranial Magnetic Stimulation59-61 and the FDA-approved external Trigeminal Nerve Stimulator.62-64
Monitoring and Long-Term Effects of ADHD
Once treatment is begun, the central question is, “Is the treatment working?” The answer to this question is not as straightforward as it may at first appear, as ADHD symptoms and the capacity to compensate for them may vary over time and with circumstance (e.g., school day or weekend, the presence of psychosocial stress), by symptom subtype (e.g., hyperactivity, inattention, impulsivity), and by functional domain (academics, risk-taking behaviors, socialization). Thus, valid and reliable methods are needed to monitor treatment response easily and accurately, but they are lacking for the majority of general clinicians. If the current treatment is not producing the desired response, or if side effects are limiting the dose of medication prescribed, the final question is what to do next to improve short- and long-term outcomes. For example, is it better to optimize dosing of the current medication, switch to another first-line medication, switch to a second-line medication, add an additional medication, or add an adjunctive psychological or behavioral therapy? And how does a clinician or parent prevent the complete abandonment of treatment, which is exceedingly common, when the first line treatment is ineffective or produces troubling side effects?65
After a child is diagnosed with ADHD, and an initial treatment strategy is determined, a monitoring strategy is applied to ensure that outcomes are evaluated over time, and modification to treatments are made when needed.66 Repeat monitoring allows intervention (e.g., change in treatment) before the final outcomes associated with ADHD occur. Several instruments are available to monitor treatment response and adverse effects over time, including the Vanderbilt scales, the Conner scales, and the SNAP-IV rating scales. Monitoring also includes assessment of any adverse effects of treatment. There are variations in the frequency of monitoring, often based on the age of the child, the specific treatment, duration of treatment, previous symptoms and comorbid conditions, and family and health care provider preferences.
Finally, one-third to one-half of patients with ADHD will have clinically significant symptoms that persist into adulthood. Co-occurring problems are the rule, as approximately half are diagnosed with an oppositional defiant or conduct disorder diagnosis, one-third have an anxiety disorder, and 20 percent have depression.2 Youth with ADHD are at increased risk for future problems associated with risk-taking, such as substance abuse, motor vehicle accidents, unprotected sexual intercourse, and criminal behavior. They are at considerable risk as adults for chronic health problems, including diabetes, heart disease, and poor oral health, in part because they engage in behaviors that increase risk for these conditions, and they often fail to adhere to health-protective behaviors. They are also at risk for future depression, anxiety, suicide attempts, and problematic peer and family relationships.44 In addition, the long-term effectiveness of standard and novel interventions for ADHD, and their potential long-term adverse effects, are not well known67-71 and are difficult to detect and document,72-74 but they are extremely important considerations for patients, parents, and clinicians as they make treatment decisions. Knowledge of the ways in which unique patient characteristics modify these short- and long-term treatment outcomes is essential to tailor and personalize care for individual patients.75
Rationale for Evidence Review
This review updates prior AHRQ reviews on ADHD.5, 47, 76 This current review builds on the previous reports and will address important gaps in knowledge related to the diagnosis of ADHD, concerns about overtreatment and undertreatment, and conflicting literature about the effectiveness of long-term treatment.
Since the last AHRQ report has been published, further diagnostic and treatment strategies have been suggested, warranting an update of the literature. Identified references address predominantly diagnostic questions such as the diagnostic validity of specific tests and suggested diagnostic tools.10, 11, 20, 23, 77 Furthermore, key studies that provide important information on the diagnosis of ADHD predate the most recent ADHD report. Hence, the current systematic review will include older studies. Searches will go back to 1980, when the diagnosis of ADHD and its diagnostic criteria were first introduced in the DSM as Attention Deficit Disorder with or without hyperactivity (DSM-III).78
In addition, since the last AHRQ review, several intervention evaluations have been published that explore different interventions such as game-based cognitive therapy or modalities such as computer training.48, 55, 63, 79-81 Furthermore, key studies that predate the most recent ADHD report provide important information on the treatment of ADHD. Hence, the current systematic review also includes older treatment studies. Searches will go back to 1980, when long acting stimulants were introduced, heralding the modern era of ADHD pharmacotherapy.
Given that the 2018 AHRQ report on ADHD identified no monitoring study, we removed the search date for this question and will aim for a comprehensive review that considers older studies (the 2018 report only included studies published to 2009). Based on discussions and preliminary literature searches, we still do not expect to identify a substantial amount of data for monitoring strategies and long-term effects, but some data may be available from the educational and school psychology literature such as Response to Intervention – Behavioral (RTI-B) strategies to monitor behavioral-based psychosocial interventions in the classroom relevant to child and adolescent ADHD outcomes.
The systematic review aims to inform a planned update of the current American Academy of Pediatrics (AAP) guidelines.
The key questions proposed for the systematic review, addressing diagnosis (Key Question 1), treatment (Key Question 2), and monitoring (Key Question 3) of ADHD, were refined following input from Key Informants, stakeholder input through public posting, and a townhall organized by the Patient-Centered Outcomes Research Institute (PCORI).
We obtained input from eight key informants. Key Informants included a parent of an underserved, ethnic minority youth with ADHD, an advocate from the national advocacy group CHADD (Children and Adults with ADHD), an expert in medical safety, an expert in testing and assessment, a representative from the Association for Child and Adolescent Counseling (ACAC), a family medicine representative, and members of the guideline group who will use the review to update the guidelines. The key informants showed strong support for the importance and relevance of the first two key questions that address the diagnosis and treatment of ADHD. They suggested relevant references and provided important input on terminology relevant to the literature searches. There were discussions about developments since the last report and about where the field is now from the perspective of each participant.
Additional input on the project was received through public posting of the review questions on the AHRQ website. The posting aimed to elicit responses from stakeholders to ensure that the review is addressing the right questions, and all aspects have been considered. A submission from the American Psychological Association (APA) and a submission from a researcher at Immaculata University addressed all review questions. For Key Question 1, input stressed the importance of minimizing false positive diagnoses from the presence of co-occurring conditions; costs and reliability of EEG diagnostic information; that a developmental lens should be adopted (e.g., does a child’s relative age and developmental maturity in comparison to classmates influence the odds of receiving a diagnosis of ADHD?); that the role of sleep, trauma, and language development should be considered; and that annual reassessments of behaviors and impairment are important. For Key Question 2, input addressed the importance of reviewing the effects of medications and the risk of diversion of pharmacological treatment; of treatment fidelity; of adherence to and persistence of medication use; of behavioral treatment, including use of different modalities (in person, video, online); and of the Multimodal Treatment of ADHD study, specifically. For Key Question 3, the input targeted the conduct of routine assessments, including reports from parents, teachers, and the children/adolescents, that should be accessible to all parties; and that routine monitoring should be part of the child/adolescent’s record.66
Finally, PCORI conducted an online townhall meeting in November 2021. There were passionate discussions and advocacy for changes in ADHD policy and research. Some participants felt strongly that both important policies and data were lacking across the board. Specific areas identified by this group included lumping ADHD-Inattentive with the Combined presentation, the lack of empirical data on executive function training and executive function coaches, the general lack of specific and feasible non-pharmacological interventions that parents can use easily and have access to, as well as the lack of availability of parent training programs being offered before initiating stimulant medication.
Following key informant and stakeholder input, the draft key questions are as follows:
- Key Question 1: For the diagnosis of ADHD:
- What is the comparative diagnostic accuracy of approaches that can be used in the primary care practice setting or by specialists to diagnose ADHD among individuals younger than 7 years of age?
- What is the comparative diagnostic accuracy of EEG, imaging, or approaches assessing executive function that can be used in the primary care practice setting or by specialists to diagnose ADHD among individuals aged 7 through 17?
- For both populations, how does the comparative diagnostic accuracy of these approaches vary by clinical setting, including primary care or specialty clinic, or patient subgroup, including age, sex, or other risk factors associated with ADHD?
- What are the adverse effects associated with being labeled correctly or incorrectly as having ADHD?
- Key Question 2: What are the comparative safety and effectiveness of pharmacologic and/or nonpharmacologic treatments of ADHD in improving outcomes associated with ADHD?
- How do these outcomes vary by presentation (inattentive, hyperactive/impulsive, and combined) or other comorbid conditions?
- What is the risk of diversion of pharmacologic treatment?
- Key Question 3: What are the comparative safety and effectiveness of different empirical monitoring strategies to evaluate the effectiveness of treatment in improving ADHD symptoms or other long-term outcomes?
While the diagnosis and treatment key questions are unchanged from the 2018 report on the topic, the key question regarding monitoring was rephrased for clarity. Of note, the restricted age range for sub-question 1b is based on recognition that most of these specialized technologies require the child to remain very still, which is difficult for children younger than seven. Neuropsychological tests as well as genetic markers are included in 1a and 1b. In question 1d, we will assess whether the literature suggests whether these adverse effects differ for those youth who are on the threshold of clinical or subclinical diagnoses. Co-morbidities include co-occurring conditions such as autism spectrum disorders, Williams syndrome, Down syndrome, learning and language disabilities, and developmental coordination disorder. Questions 2 and 3 will review effectiveness as well as adverse outcomes.
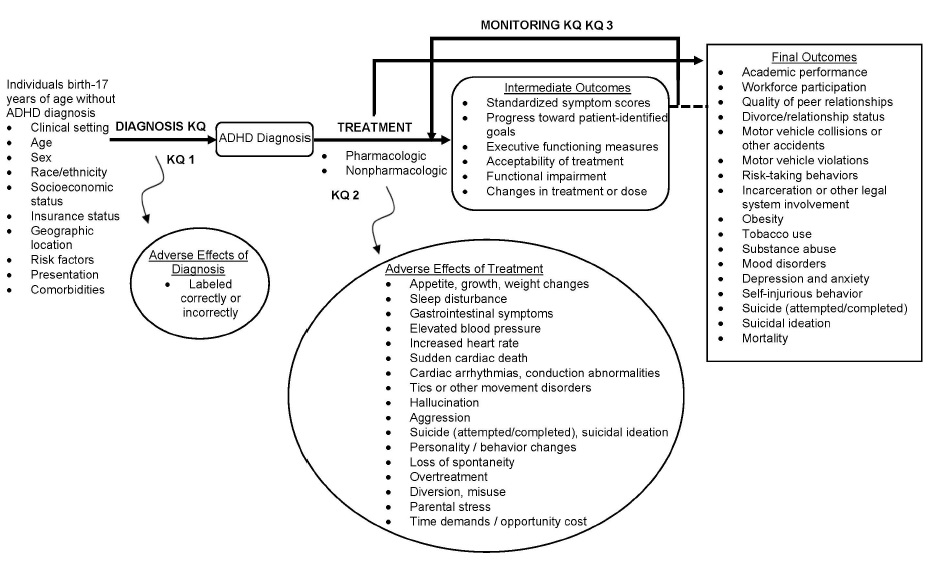
The analytic framework depicts the key questions and outcomes to evaluate the diagnosis, treatment, and monitoring strategies for ADHD.
Figure 1: Analytic Framework
Criteria for Inclusion/Exclusion of Studies in the Review
The eligibility criteria are organized in a PICOTSO (population, intervention, comparator, outcome, timing, setting, study design, and other limiters) framework. The eligibility criteria are unchanged from the prior report, with the exception of now including further tests and treatments published before and after the 2018 ADHD report. In addition, randomized controlled trials (RCTs) are no longer limited by sample size given that RCTs allow strong evidence statements; however, treatment studies with fewer than 100 participants will be required to report a power calculation to ensure that the studies had sufficient statistical power to detect a difference between the intervention and control or comparator group. Finally, no comparator is needed anymore for studies reporting on long-term effects, and these studies are not restricted by publication date, given the small evidence base.
Table 1. Eligibility Criteria
| PICOTS Element | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
|
Population |
KQ 1 (diagnosis): Individuals birth through 17 years of age without the diagnosis of ADHD |
KQ1, KQ2: Individuals 18 years of age or older unless findings are reported separately for individuals 18 years and under, or if the mean patient age plus the standard deviation is not greater than 21 years of age |
|
Interventions |
KQ 1 (diagnosis): Any standard ADHD diagnostic strategy, including clinician interview, standardized instrument (e.g., Vanderbilt scales, Conner scales, SNAP-IV rating score), neuropsychological test measures (e.g., working memory, processing speed, continuous performance tasks) for individuals under 7 years of age. The use of EEG-based systems, imaging, or assessment of executive function for the diagnosis of ADHD in individuals through 17 years
KQ 3 (monitoring): Follow-up visits in primary care using various methods and frequencies (monthly to annually) for monitoring, independent of treatment, including the selection of scales/validated tools for monitoring of ADHD severity and treatment response along with forms of remote monitoring or telehealth strategies |
KQ 1: Validation studies or diagnosis conducted using a non-validated instrument KQ 2: Studies with less than 4 weeks of treatment |
|
Comparators |
KQ 1 (diagnosis): Confirmation of diagnosis by a specialist (gold standard), such as a psychologist, psychiatrist or other care provider using a well-validated and reliable process of confirming the diagnosis of ADHD according to the DSM-5 |
KQ 1: Comparison to diagnosis with a non-validated instrument |
|
Outcomes |
KQ1 (diagnosis):
KQ 2 (treatment):
KQ 3 (monitoring):
|
|
|
Timing |
KQ 1 (diagnosis):
KQ 2 (treatment) and KQ 3 (monitoring): Any |
|
|
Setting |
KQ 1 (diagnosis): Primary or specialty care settings |
|
|
Study Design |
KQ1-3: Randomized controlled trials (RCTs) |
Editorials, nonsystematic reviews, letters, case series, case reports, pre-post studies. Because small studies are often pilot studies or studies of lower quality, these are excluded. Systematic reviews are not eligible for inclusion but will be retained. |
|
Other limiters |
|
Non-English language and abbreviated publications (abstracts, letters) |
Note: FDA: Food and Drug Administration, KQ: Key Question
Relevant systematic reviews and meta-analyses will be retained as background or for reference-mining but will not be included as evidence. Publications reporting on the same participants will be consolidated into one study record. Reports exclusively published in non-English language publications remain excluded given the high volume of literature, the focus on the review on populations in the U.S., the scope of the key questions, and the aim to support a U.S. clinical practice guideline.
Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies to Answer the Key Questions
For primary research studies, we will search the database PubMed (biomedical literature), EMBASE (pharmacology emphasis), PsycINFO (psychological research), and ERIC (education research). We will also search the U.S. trial database – ClinicalTrials.gov – to capture all relevant data regardless of the publication status. Increasingly trial registries include data and a complete record of adverse events, making them an important evidence review tool to identify all relevant data and to reduce publication bias.
We will also use existing reviews for reference-mining; these will be identified through the same databases used for primary research plus searching the Cochrane Database of Systematic Reviews, Campbell Collaboration, What Works in Education, and PROSPERO. Scoping searches identified several published reviews. These often address medication treatment with an increased focus on safety.82-86 Given that many practice guidelines are now based on systematic reviews, we will also search the ECRI Guidelines Trust, G-I-N, and ClinicalKey. Using external systematic reviews in addition to building on the 2018 AHRQ report will increase the certainty that all relevant studies have been captured.
The literature searches for this project will build on all prior ADHD reports published by AHRQ:
- Key Question 1: Searches will cover 1980 to 2011, and 2016 to present. Since research published between 2011 and 2016 was thoroughly screened by the 2018 review, we will use the identified studies listed in the 2018 AHRQ report to cover 2011 to 2016.
- Key Question 2: Searches will cover 1980 to 2011, omitting search terms covered in the 2011 AHRQ report and adding the adolescent population, which was not previously fully covered. Searches will also cover 2016 to present. Since research published between 2011 and 2016 was thoroughly screened by the 2018 review, we will use the identified studies listed in the 2018 AHRQ report.
- Key Question 3: Searches will not be limited by date. The search strategies will be similar to the 2018 report; however, we will update the searches due to database changes and new diagnostic and treatment approaches.
The basic draft literature strategy for the database PubMed is shown in the appendix. The complex search strategy will undergo peer review to ensure high quality searches.
In addition, we will update the meta-analyses included in the prior report and incorporate prior and new studies into pooled analyses in order to provide a full picture of the existing evidence.5, 47 Furthermore, we will use information provided by content experts,87 and the technical expert panel will review the list of included studies to ensure that all relevant literature has been captured.
We will use detailed pre-established criteria to determine eligibility for inclusion and exclusion of publications in accordance with the AHRQ Methods Guide for Effectiveness and Comparative Effectiveness Reviews. To reduce reviewer errors and bias, all citations will be reviewed by a human reviewer and a machine learning algorithm. Citations deemed potentially relevant will be obtained as full text. Each full-text article will be independently reviewed for eligibility by two literature reviewers, including any articles suggested by peer reviewers or that arise from the public posting process, submission through the SEADS portal, or response to Federal Register notice. Any disagreements will be resolved by consensus. We will maintain a record of studies excluded at the full-text level with reasons for exclusion.
While the draft report is under peer review and open for public comment, we will update the search and include any eligible studies identified either during that search or through peer or public reviews in the final report.
Data Abstraction and Data Management
Using established templates from the 2018 review, data from new studies will be abstracted regarding study details, methods, and results. We will capture the same information for the new studies that have been collected for the old studies so that all analyses can be updated.
The review team will create data abstraction forms for the key questions in DistillerSR, an online program for systematic reviews. Forms include detailed guidance to support reviewers to aid both reproducibility and standardization of data collection. Based on their clinical and methodological expertise, researchers will be assigned to abstract data from each of the eligible articles. One researcher will abstract the data, and a second reviewer will check for accuracy and completeness. Disagreements will be resolved by consensus.
We will design the data abstraction forms for this project to collect the data required to evaluate the study, as well as demographic and other data needed for determining outcomes (i.e., intermediate, final, and adverse events outcomes). We will pay particular attention to describing the details of the treatment (e.g., pharmacotherapy dosing, methods of behavioral interventions), patient characteristics (e.g., ADHD presentation, comorbidities, age), and study design (e.g., RCT versus observational) that may be related to outcomes. In addition, we will carefully describe comparators, as treatment standards may have changed during the period covered by the review. The safety outcomes will be framed to help identify adverse events, including those from drug therapies and those resulting from misdiagnosis and labeling.
We will discuss the best classification of mixed samples (e.g., studies not restricted to children under 7 years of age), informed by pragmatic solutions from prior work.88 We will adapt some of the categories to capture promising development such as SMART designs and will add selected variables based on our experience with nutrition interventions.89-91 In addition, we will contact authors to identify subgroup data for young children relevant to KQ1.
Data necessary for assessing quality and applicability as described in the EPC Methods Guide will also be abstracted. FormsGrading the Strength of Evidence (SOE) for Major Comparisons and Outcomes will be pilot-tested with a sample of included articles to ensure that all relevant data elements are captured and that ambiguity is avoided. Final abstracted data will be uploaded to SRDR per EPC requirements.
Assessment of Methodological Risk of Bias of Individual Studies
The critical appraisal for individual studies will apply criteria consistent with QUADAS 2 for diagnostic studies and the RoB 2 guidance for common sources of bias in intervention studies adapted for the eligible study designs.92, 93
QUADAS 2 evaluates four domains: patient selection, index test characteristics, reference standard quality, as well as flow and timing93:
- Patient selection: The domain patient selection addresses whether the selection of patients could have introduced bias, taking into account whether the study enrolled a consecutive or random sample, whether the data are not based on a retrospective case-control design, and whether the study avoided inappropriate or problematic exclusions from the patient pool.
- Index test: The index test domain evaluates whether the conduct or interpretation of the test could have introduced bias, taking into account whether the results of the test were interpreted without knowledge of the results of the reference standard and whether any thresholds or cut-offs were pre-specified (e.g., instead of determined in the study to maximize diagnostic performance).
- Reference standard: The domain reference standard evaluates whether the reference standard, its conduct, or its interpretation may have introduced bias, taking into account the quality of the reference standard in correctly classifying the condition (e.g., a gold standard may not exist) and whether the reference standard test results were interpreted without knowledge of the results or index test.
- Flow and timing: The last domain, flow and timing, evaluates whether the conduct of the study may have introduced bias. The assessment takes into account whether the interval between the test and the reference standard was appropriate, whether all patients received the reference standard and whether they received the same reference standard, and whether all patients were included in the analysis. For each domain, we assessed the potential risk of bias in the study in order to identify high risk of bias and low risk of bias studies. Consistent with QUADAS-2,93 the critical appraisal will evaluate for each study and appraisal domain whether there are concerns regarding the applicability of the study results to the review question. This encompassed whether the patients included in the studies do not match the review question; whether the test, its conduct, or interpretation differ from the review question; or whether the target condition as defined by the reference standard does not fully match the review question.
The adapted risk of bias tool will assess selection, detection, performance, attrition, reporting, and study-specific sources of bias:
- Selection bias: For selection bias, we will assess the randomization sequence and allocation concealment in RCTs as well as baseline differences and potential confounders in all studies.
- Performance bias: Performance bias will evaluate whether patient- or caregiver knowledge of the intervention allocation or circumstances such as the trial context may have affected the outcome, and whether any deviations from intended interventions were balanced between groups.
- Attrition bias: Attrition bias will consider the number of dropouts, any imbalances across study arms, and whether missing values may have affected the reported outcomes.
- Detection bias: Detection bias will assess whether outcome assessors were aware of the intervention allocation, whether this knowledge could have influenced the outcome measurement, and whether the outcome ascertainment could differ between arms.
- Reporting bias: Reporting bias assessment will include an evaluation of whether a pre-specified analysis plan exists (e.g., a published protocol), whether the numerical results likely have been selected on the basis of the results, and whether key outcomes were not reported (e.g., an obvious effectiveness indicator is missing) or inadequately reported (e.g., anecdotal adverse event reporting).
- Study-specific sources of bias: In addition to the types of bias listed above, we will assess other potential sources of bias such as early termination of studies, inadequate reporting of intervention details, and lack of intention-to-treat analyses.
We will incorporate the risk of bias result into the strength of evidence assessment and downgrade out confidence in evidence summaries in the presence of study limitations.
Data Synthesis
We will begin by summarizing key features of the included studies, addressing study design; participant characteristics; settings; diagnostic, treatment, and monitoring strategies; and intermediate, final, and adverse event outcomes. We will answer each key question with the available evidence. We will order our findings by diagnostic, treatment, and monitoring strategy and comparators, and then within these comparisons, by outcome with long-term final outcomes emphasized.
We will determine the feasibility of a quantitative synthesis (i.e., meta-analysis). Feasibility depends on the volume of relevant literature, conceptual homogeneity of the studies, and completeness of the reporting of results. When a meta-analysis is appropriate, we will use random-effects models corrected for small numbers of studies where necessary to synthesize the available evidence quantitatively.94 In addition, we will update meta-analyses included in the 2018 report to summarize data and obtain more precise estimates in cumulative analyses. We will present summary estimates and 95 percent confidence intervals. We will test for heterogeneity using graphical displays and the I-squared statistics. We will explore potential sources of heterogeneity while recognizing that the ability of statistical methods to detect heterogeneity may be limited.
We anticipate that intervention effects may be heterogeneous. We hypothesize that the methodological rigor of individual studies, study type, the characteristics of the comparator, and patients’ underlying clinical presentation are potentially associated with the intervention effects. If there are sufficient studies, we will perform meta-regression analyses to examine these hypotheses. Pre-defined subgroups include children younger than 7 years of age; children and adolescents, 7 through 11; youth, 12 through 17; as well as subgroups by sex/gender. In addition, we will assess the effect of treatment and diagnosis in participants with concomitant learning disabilities; the racial and ethnic composition of study samples; the personnel involved; and the potential effect of the diagnostic, treatment, and monitoring setting in meta-regressions across studies.
Grading the Strength of Evidence (SOE) for Major Comparisons and Outcomes
The strength of evidence assessment will clearly document uncertainty, outline the reasons for insufficient evidence where appropriate, and communicate our confidence in the findings.
The strength of evidence for each body of evidence (based on the Key Question, diagnostic and treatment approach, comparator, and outcome) will be initially assessed by one researcher with experience in determining strength of evidence for each primary clinical outcome by following the principles for adapting GRADE (Grading of Recommendations Assessment, Development and Evaluation), outlined in the AHRQ methods guide.95 The initial assessment will be discussed in the team.
We prioritized outcomes with the help of the Technical Expert Panel (TEP) in combination with team expertise. We considered outcomes most clinically relevant and important to patients and clinicians to guide clinical practice. The following outcomes were selected for the strength of evidence assessment:
- Key question 1: Sensitivity, specificity, costs, inter-rater reliability, internal consistency, test-retest reliability, misdiagnosis (risk of missed condition that can appear as ADHD).
- Key question 2: Behavior changes, broad-band scale scores describing behavior, standardized symptom scores, functional impairment, acceptability of treatment, academic rating scale scores, appetite changes and growth suppression, number of participants with adverse events.
- Key question 3: Functional impairment, broad-band scale scores, standardized symptom scores, progress toward patient-identified goals, acceptability of treatment, academic rating scale scores, any long-term effects, growth suppression, quality of peer relationships.
In determining the strength of a body of evidence, the following domains will be evaluated:
- Study limitations: The extent to which studies reporting on a particular outcome are likely to be protected from bias. The aggregate risk of bias across individual studies reporting an outcome is considered; graded as low, medium, or high level of study limitations
- Inconsistency: The extent to which studies report the same direction or magnitude of effect for a particular outcome; graded as consistent, inconsistent, or unknown (in the case of a single study)
- Indirectness: Generally reflects whether the outcome is directly or indirectly related to health outcomes of interest. Patient-centered outcomes are considered direct. Comparisons of an intervention to placebo or usual care are considered indirect; graded as direct or indirect.
- Imprecision: Describes the level of certainty of the estimate of effect for a particular outcome, where a precise estimate is one that allows a clinically useful conclusion. Graded as precise or imprecise. When quantitative synthesis is not possible, sample size and assessment of variance within individual studies will be considered.
- Reporting bias: Occurs when publication or reporting of findings is based on their direction or magnitude of effect. Publication bias, selective outcome reporting, and selective analysis reporting are types of reporting bias. Reporting bias is difficult to assess as systematic identification of unpublished evidence is challenging. If sufficient numbers of RCTs are available, we will review Begg and Egger tests.
Bodies of evidence consisting of RCTs are initially considered as high strength, while bodies of comparative observational studies begin as low-strength evidence. The strength of the evidence may be downgraded based on the limitations described above. There are also situations where observational evidence may be upgraded (e.g., large magnitude of effect, presence of dose-response relationship or existence of plausible unmeasured confounders) as described in the AHRQ Methods guides.95
A final strength of evidence grade will be assigned by evaluating and weighing the combined results of the above domains. To ensure consistency and validity of the evaluation, the grades will be reviewed by the entire team of investigators. The strength of evidence will be assigned an overall grade of high, moderate, low, or insufficient according to a four-level scale:
- High: We are very confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. We believe that the findings are stable (i.e., another study would not change the conclusions).
- Moderate: We are moderately confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has some deficiencies. We believe that the findings are likely to be stable, but some doubt remains.
- Low: We have limited confidence that the estimate of effect lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). We believe that additional evidence is needed before concluding either that the findings are stable or that the estimate of effect is close to the true effect.
- Insufficient: We have no evidence, we are unable to estimate an effect, or we have no confidence in the estimate of effect for this outcome. No evidence is available, or the body of evidence has unacceptable deficiencies, precluding reaching a conclusion.
Summary tables will include ratings for individual strength of evidence domains (i.e., risk of bias, consistency, precision, directness) based on the totality of underlying evidence (i.e., the existing evidence included in the prior report in combination with newly identified studies). We will summarize updated evidence and describe what it adds to the previous review and highlight changes to the key findings.
Assessing Applicability
Applicability will be assessed in accordance with the AHRQ's Methods Guide. Factors that may affect applicability, which we have identified a priori, include patient, intervention, comparisons, outcomes, settings, and study design features.
Consistent with the prior AHRQ report, we will address whether outcomes are different across studies that recruit different populations (e.g., age groups, ADHD presentations, exclusions for comorbidities) or use different methods to implement the interventions of interest. We will use these data to evaluate the applicability to clinical practice, paying special attention to the following: study eligibility criteria; demographic features of the enrolled population in comparison to the target population; characteristics of the intervention used in comparison with care models currently in use; the possibility of diagnostic tool or treatment intervention learning curves; and clinical relevance and timing of the outcome measures. We will use this information to assess the situations in which the evidence is most relevant and to evaluate applicability to real-world clinical practice in typical U.S. settings, summarizing applicability assessments qualitatively.
- Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. Journal of Clinical Child and Adolescent Psychology. 2018 Mar-Apr;47(2):199-212. doi: 10.1080/15374416.2017.1417860. PMID: 29363986.
- Center for Disease Control and Prevention. Data and Statistics About ADHD. Accessed August 23, 2021.
- Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007 Jun;164(6):942-8. doi: 10.1176/ajp.2007.164.6.942. PMID: 17541055.
- Hong SB, Dwyer D, Kim JW, et al. Subthreshold attention-deficit/hyperactivity disorder is associated with functional impairments across domains: a comprehensive analysis in a large-scale community study. Eur Child Adolesc Psychiatry. 2014 Aug;23(8):627-36. doi: 10.1007/s00787-013-0501-z. PMID: 24318039.
- Charach A, Dashti B, Carson P, et al. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment. Compartative Effectiveness Review No. 44 (Prepared by the McMaster University Evidence-based Practice Center under Contract No. MME2202 290-02-0020.) AHRQ Publication No. 12-EHC003-EF Agency for Healthcare Research and Quality Rockville, MD: Oct Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment October 2011.
- Shi Y, Hunter Guevara LR, Dykhoff HJ, et al. Racial Disparities in Diagnosis of Attention-Deficit/Hyperactivity Disorder in a US National Birth Cohort. JAMA Netw Open. 2021 Mar 1;4(3):e210321. doi: 10.1001/jamanetworkopen.2021.0321. PMID: 33646315.
- Morgan PL, Hillemeier MM, Farkas G, et al. Racial/ethnic disparities in ADHD diagnosis by kindergarten entry. J Child Psychol Psychiatry. 2014 Aug;55(8):905-13. doi: 10.1111/jcpp.12204. PMID: 24456307.
- Fadus MC, Ginsburg KR, Sobowale K, et al. Unconscious Bias and the Diagnosis of Disruptive Behavior Disorders and ADHD in African American and Hispanic Youth. Academic Psychiatry. 2020 2020/02/01;44(1):95-102. doi: 10.1007/s40596-019-01127-6.
- Chan E, Hopkins MR, Perrin JM, et al. Diagnostic practices for attention deficit hyperactivity disorder: a national survey of primary care physicians. Ambul Pediatr. 2005 Jul-Aug;5(4):201-8. doi: 10.1367/A04-054R1.1. PMID: 16026184.
- Nobel E, Brunnekreef JA, Schachar RJ, et al. Parent–clinician agreement in rating the presence and severity of attention-deficit/hyperactivity disorder symptoms. ADHD Attention Deficit and Hyperactivity Disorders. 2019 2019/03/01;11(1):21-9. doi: 10.1007/s12402-018-0267-8. PMID: 30927229.
- Parker A, Corkum P. ADHD Diagnosis:As Simple As Administering a Questionnaire or a Complex Diagnostic Process? Journal of Attention Disorders. 2016 Jun;20(6):478-86. doi: 10.1177/1087054713495736. PMID: 23887860.
- Chang L-Y, Wang M-Y, Tsai P-S. Diagnostic Accuracy of Rating Scales for Attention-Deficit/Hyperactivity Disorder: A Meta-analysis. Pediatrics. 2016 Mar;137(3):e20152749. doi: 10.1542/peds.2015-2749. PMID: 26928969.
- Asherson P, Trzaskowski M. Attention-deficit/hyperactivity disorder is the extreme and impairing tail of a continuum. J Am Acad Child Adolesc Psychiatry. 2015 Apr;54(4):249-50. doi: 10.1016/j.jaac.2015.01.014. PMID: 25791141.
- Greven CU, Merwood A, van der Meer JMJ, et al. The opposite end of the attention deficit hyperactivity disorder continuum: genetic and environmental aetiologies of extremely low ADHD traits. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2016;57:523 - 31.
- McLennan JD. Understanding attention deficit hyperactivity disorder as a continuum. Canadian family physician Medecin de famille canadien. 2016;62(12):979-82. PMID: 27965331.
- De Crescenzo F, Licchelli S, Ciabattini M, et al. The use of actigraphy in the monitoring of sleep and activity in ADHD: A meta-analysis. Sleep Med Rev. 2016 Apr;26:9-20. doi: 10.1016/j.smrv.2015.04.002. PMID: 26163053.
- Williams LM, Hermens DF, Thein T, et al. Using Brain-Based Cognitive Measures to Support Clinical Decisions in ADHD. Pediatric Neurology. 2010 2010/02/01/;42(2):118-26. doi: https://doi.org/10.1016/j.pediatrneurol.2009.08.010. PMID: 20117748.
- Wasserman T, Wasserman LD. The Sensitivity and Specificity of Neuropsychological Tests in the Diagnosis of Attention Deficit Hyperactivity Disorder. Applied Neuropsychology: Child. 2012 2012/07/01;1(2):90-9. doi: 10.1080/21622965.2012.702025. PMID: 23428295.
- Barkley RA. Neuropsychological Testing is Not Useful in the Diagnosis of ADHD: Stop It (or Prove It)! The ADHD Report. 2019;27(2):1-8. doi: 10.1521/adhd.2019.27.2.1.
- Hult N, Kadesjö J, Kadesjö B, et al. ADHD and the QbTest: Diagnostic Validity of QbTest. Journal of Attention Disorders. 2018 Sep;22(11):1074-80. doi: 10.1177/1087054715595697. PMID: 26224575.
- Anita Thapar, F.R.C.Psych., Ph.D. Discoveries on the Genetics of ADHD in the 21st Century: New Findings and Their Implications. American Journal of Psychiatry. 2018 Oct 1;175(10):943-50. doi: 10.1176/appi.ajp.2018.18040383. PMID: 30111187.
- Snyder SM. Systems and methods to identify a subgroup of ADHD at higher risk for complicating conditions. US Patent and Trademark Office. (U.S. PPA Number 61/237,911; August 27, 2009) (U.S. PA Number 12/870,328; August 28, 2010). 2010.
- Snyder SM, Rugino TA, Hornig M, et al. Integration of an EEG biomarker with a clinician's ADHD evaluation. Brain and Behavior. 2015 Apr;5(4):e00330. doi: https://doi.org/10.1002/brb3.330. PMID: 25798338.
- Bansal R, Staib LH, Laine AF, et al. Anatomical brain images alone can accurately diagnose chronic neuropsychiatric illnesses. PLoS One. 2012;7(12):e50698. doi: 10.1371/journal.pone.0050698. PMID: 23236384.
- Haubold A, Peterson BS, Bansal R. Annual research review: progress in using brain morphometry as a clinical tool for diagnosing psychiatric disorders. J Child Psychol Psychiatry. 2012 May;53(5):519-35. doi: 10.1111/j.1469-7610.2012.02539.x. PMID: 22394424.
- Ford-Jones PC. Misdiagnosis of attention deficit hyperactivity disorder: 'Normal behaviour' and relative maturity. Paediatrics & child health. 2015 May;20(4):200-2. doi: 10.1093/pch/20.4.200. PMID: 26038639.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: Psychiatric Publishing; 2013.
- Sciutto MJ, Eisenberg M. Evaluating the Evidence For and Against the Overdiagnosis of ADHD. Journal of Attention Disorders. 2007 Sep;11(2):106-13. doi: 10.1177/1087054707300094. PMID: 17709814.
- Tseregounis IE, Stewart SL, Crawford A, et al. Age- and Sex-Specific Increases in Stimulant Prescribing Rates—California, 2008-2017. Journal of Attention Disorders. 2020 Jan;24(2):205-14. doi: 10.1177/1087054719883008. PMID: 31680608.
- Board AR, Guy G, Jones CM, et al. Trends in stimulant dispensing by age, sex, state of residence, and prescriber specialty — United States, 2014–2019. Drug and Alcohol Dependence. 2020 2020/12/01/;217:108297. doi: https://doi.org/10.1016/j.drugalcdep.2020.108297. PMID: 32961454.
- Kazda L, Bell K, Thomas R, et al. Overdiagnosis of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: A Systematic Scoping Review. JAMA Network Open. 2021 Apr 1;4(4):e215335-e. doi: 10.1001/jamanetworkopen.2021.5335. PMID: 33843998.
- Cook G. Big Pharma’s Manufactured Epidemic: The Misdiagnosis of ADHD. Scientific American.Oct 11, 2016.
- Cha AE. CDC warns that Americans may be overmedicating youngest children with ADHD. The Washington Post. 2016.
- Ramachandran S, Dertien D, Bentley SI. Prevalence of ADHD symptom malingering, nonmedical use, and drug diversion among college-enrolled adults with a prescription for stimulant medications. Journal of Addictive Diseases. 2020 2020/02/17;38(2):176-85. doi: 10.1080/10550887.2020.1732762. PMID: 32242510.
- Visser SN, Danielson ML, Bitsko RH, et al. Trends in the Parent-Report of Health Care Provider-Diagnosed and Medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003-2011. Journal of the American Academy of Child & Adolescent Psychiatry. 2014 Jan;53(1):34-46.e2. doi: 10.1016/j.jaac.2013.09.001. PMID: 24342384.
- DosReis S, Barksdale CL, Sherman A, et al. Stigmatizing experiences of parents of children with a new diagnosis of ADHD. Psychiatr Serv. 2010 Aug;61(8):811-6. doi: 10.1176/ps.2010.61.8.811. PMID: 20675840.
- Cook J, Knight E, Hume I, et al. The self-esteem of adults diagnosed with attention-deficit/hyperactivity disorder (ADHD): a systematic review of the literature. Atten Defic Hyperact Disord. 2014 Dec;6(4):249-68. doi: 10.1007/s12402-014-0133-2. PMID: 24668198.
- Lebowitz MS. Stigmatization of ADHD: A Developmental Review. J Atten Disord. 2016 Mar;20(3):199-205. doi: 10.1177/1087054712475211. PMID: 23407279.
- Wiener J, Malone M, Varma A, et al. Children’s Perceptions of Their ADHD Symptoms:Positive Illusions, Attributions, and Stigma. Canadian Journal of School Psychology. 2012;27(3):217-42. doi: 10.1177/0829573512451972.
- Weinstein D, Staffelbach D, Biaggio M. Attention-deficit hyperactivity disorder and posttraumatic stress disorder: Differential diagnosis in childhood sexual abuse. Clinical Psychology Review. 2000 2000/04/01/;20(3):359-78. doi: https://doi.org/10.1016/S0272-7358(98)00107-X. PMID: 10779899.
- Szymanski K, Sapanski L, Conway F. Trauma and ADHD – Association or Diagnostic Confusion? A Clinical Perspective. Journal of Infant, Child, and Adolescent Psychotherapy. 2011 2011/01/01;10(1):51-9. doi: 10.1080/15289168.2011.575704.
- Langevin R, Marshall C, Wallace A, et al. Disentangling the Associations Between Attention Deficit Hyperactivity Disorder and Child Sexual Abuse: A Systematic Review. Trauma, Violence, & Abuse. 2021 Jul 9:15248380211030234. doi: 10.1177/15248380211030234. PMID: 34238078.
- Dahmen B, Pütz V, Herpertz-Dahlmann B, et al. Early pathogenic care and the development of ADHD-like symptoms. Journal of Neural Transmission. 2012 2012/09/01;119(9):1023-36. doi: 10.1007/s00702-012-0809-8. PMID: 22661337.
- Erskine HE, Norman RE, Ferrari AJ, et al. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2016 Oct;55(10):841-50. doi: 10.1016/j.jaac.2016.06.016. PMID: 27663939.
- Mehta T, Mannem N, Yarasi NK, et al. Biomarkers for ADHD: the Present and Future Directions. Current Developmental Disorders Reports. 2020 2020/09/01;7(3):85-92. doi: 10.1007/s40474-020-00196-9.
- Faraone SV, Bonvicini C, Scassellati C. Biomarkers in the Diagnosis of ADHD – Promising Directions. Current Psychiatry Reports. 2014 2014/10/10;16(11):497. doi: 10.1007/s11920-014-0497-1.
- Kemper AR, Maslow GR, Hill S, et al. Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents. Comparative Effectiveness Review No. 203. (Prepared by the Duke University Evidence-based Practice Center under Contract No. 290-2015-00004-I.) AHRQ Publication No. 18-EHC005-EF Agency for Healthcare Research and Quality (US). Rockville (MD): 2018.
- Pelham WE, Jr., Fabiano GA, Waxmonsky JG, et al. Treatment sequencing for childhood ADHD: a multiple-randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol. 2016 Jul-Aug;45(4):396-415. doi: 10.1080/15374416.2015.1105138. PMID: 26882332.
- Lei H, Nahum-Shani I, Lynch K, et al. A "SMART" design for building individualized treatment sequences. Annual review of clinical psychology. 2012;8:21-48. doi: 10.1146/annurev-clinpsy-032511-143152. PMID: 22224838.
- Almirall D, Nahum-Shani I, Sherwood NE, et al. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational behavioral medicine. 2014 Sep;4(3):260-74. doi: 10.1007/s13142-014-0265-0. PMID: 25264466.
- Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005 May 30;24(10):1455-81. doi: 10.1002/sim.2022. PMID: 15586395.
- Ogbagaber SB, Karp J, Wahed AS. Design of sequentially randomized trials for testing adaptive treatment strategies. Stat Med. 2016 Mar 15;35(6):840-58. doi: 10.1002/sim.6747. PMID: 26412033.
- Veloso A, Vicente SG, Filipe MG. Effectiveness of cognitive training for school-aged children and adolescents with Attention Deficit/Hyperactivity Disorder: a systematic review. Frontiers in Psychology. 2020 2020-January-14;10(2983):2983. doi: 10.3389/fpsyg.2019.02983. PMID: 32010026.
- Moore AL, Carpenter DM, 2nd, Miller TM, et al. Clinician-delivered cognitive training for children with attention problems: effects on cognition and behavior from the ThinkRx randomized controlled trial. Neuropsychiatric disease and treatment. 2018;14:1671-83. doi: 10.2147/NDT.S165418. PMID: 29983567.
- Bikic A, Leckman JF, Christensen TØ, et al. Attention and executive functions computer training for attention-deficit/hyperactivity disorder (ADHD): results from a randomized, controlled trial. European Child & Adolescent Psychiatry. 2018 2018/12/01;27(12):1563-74. doi: 10.1007/s00787-018-1151-y. PMID: 29644473.
- Scionti N, Cavallero M, Zogmaister C, et al. Is cognitive training effective for improving executive functions in preschoolers? a systematic review and meta-analysis. Frontiers in Psychology. 2020 2020-January-10;10(2812):2812. doi: 10.3389/fpsyg.2019.02812. PMID: 31998168.
- US Food and Drug Administration. FDA Permits Marketing of First Game-Based Digital Therapeutic to Improve Attention Function in Children with ADHD. FDA News Release. 2020;June 15.
- Westwood S, Radua J, Rubia K. Non-invasive brain stimulation as an alternative treatment for ADHD: a systematic review and meta-analysis. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2019;12(2):502. doi: 10.1016/j.brs.2018.12.644.
- Zangen A. T033 Right prefrontal rTMS for the treatment of ADHD: Electrophysiological correlates and prognostic biomarkers. Clinical Neurophysiology. 2017 2017/03/01/;128(3):e11. doi: https://doi.org/10.1016/j.clinph.2016.10.131.
- Rubia K. Precision medicine in neurotherapeutics for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2021 Jul;60(7):813-5. doi: 10.1016/j.jaac.2020.11.013. PMID: 33264662.
- Wong HC, Zaman R. Neurostimulation in treating ADHD. Psychiatr Danub. 2019 Sep;31(Suppl 3):265-75. PMID: 31488739.
- Voelker R. Trigeminal nerve stimulator for ADHD. JAMA. 2019 Jun 4;321(21):2066-. doi: 10.1001/jama.2019.6992. PMID: 31162556.
- McGough JJ, Sturm A, Cowen J, et al. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2019 Apr;58(4):403-11.e3. doi: 10.1016/j.jaac.2018.11.013. PMID: 30768393.
- Abrams Z. A new device for treating ADHD in children. Monitor on Psychology. 2019;50(7).
- Miller AR, Lalonde CE, McGrail KM. Children's persistence with methylphenidate therapy: a population-based study. Can J Psychiatry. 2004 Nov;49(11):761-8. doi: 10.1177/070674370404901107. PMID: 15633854.
- Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD Clinical Care Pathway. Child Adolesc Psychiatry Ment Health. 2015;9:52. doi: 10.1186/s13034-015-0083-2. PMID: 26587055.
- Steven R. Pliszka, M.D. Is There Long-Term Benefit From Stimulant Treatment for ADHD? American Journal of Psychiatry. 2019 Sep 1;176(9):685-6. doi: 10.1176/appi.ajp.2019.19070681. PMID: 31474129.
- Craig SG, Davies G, Schibuk L, et al. Long-Term Effects of Stimulant Treatment for ADHD: What Can We Tell Our Patients? Current Developmental Disorders Reports. 2015 2015/03/01;2(1):1-9. doi: 10.1007/s40474-015-0039-5.
- Swanson JM. Debate: Are Stimulant Medications for Attention-Deficit/Hyperactivity Disorder Effective in the Long Term? (Against). J Am Acad Child Adolesc Psychiatry. 2019 Oct;58(10):936-8. doi: 10.1016/j.jaac.2019.07.001. PMID: 31515165.
- Coghill D. Debate: Are Stimulant Medications for Attention-Deficit/Hyperactivity Disorder Effective in the Long Term? (For). J Am Acad Child Adolesc Psychiatry. 2019 Oct;58(10):938-9. doi: 10.1016/j.jaac.2019.07.002. PMID: 31515164.
- Krinzinger H, Hall CL, Groom MJ, et al. Neurological and psychiatric adverse effects of long-term methylphenidate treatment in ADHD: A map of the current evidence. Neuroscience & Biobehavioral Reviews. 2019 2019/12/01/;107:945-68. doi: https://doi.org/10.1016/j.neubiorev.2019.09.023. PMID: 31545988.
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med. 2012 Sep 4;10:99. doi: 10.1186/1741-7015-10-99. PMID: 22947230.
- Arnold LE, Hodgkins P, Caci H, et al. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS One. 2015;10(2):e0116407. doi: 10.1371/journal.pone.0116407. PMID: 25714373.
- Swanson JM, Rommelse N, Cotton J, et al. 142. Attention Deficit Hyperactivity Disorder (updated chapter). In press (2022).
- Wong HK, Tiffin PA, Chappell MJ, et al. Personalized Medication Response Prediction for Attention-Deficit Hyperactivity Disorder: Learning in the Model Space vs. Learning in the Data Space. Frontiers in physiology. 2017;8:199-. doi: 10.3389/fphys.2017.00199. PMID: 28443027.
- Jadad AR, Boyle M, Cunningham C, et al. Treatment of attention-deficit/hyperactivity disorder. Evid Rep Technol Assess (Summ). 1999 Nov(11):i-viii, 1-341. PMID: 10790990.
- Vaidya CJ, You X, Mostofsky S, et al. Data-driven identification of subtypes of executive function across typical development, attention deficit hyperactivity disorder, and autism spectrum disorders. J Child Psychol Psychiatry. 2020 Jan;61(1):51-61. doi: 10.1111/jcpp.13114. PMID: 31509248.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980.
- Rajabi S, Pakize A, Moradi N. Effect of combined neurofeedback and game-based cognitive training on the treatment of ADHD: A randomized controlled study. Applied Neuropsychology: Child. 2020 2020/07/02;9(3):193-205. doi: 10.1080/21622965.2018.1556101. PMID: 30734583.
- Pelham WE, Jr., Meichenbaum DL, Smith BH, et al. Acute Effects of MPH on the Parent-Teen Interactions of Adolescents With ADHD. J Atten Disord. 2017 Jan;21(2):158-67. doi: 10.1177/1087054713480833. PMID: 23543401.
- Hannesdottir DK, Ingvarsdottir E, Bjornsson A. The OutSMARTers Program for Children With ADHD. J Atten Disord. 2017 Feb;21(4):353-64. doi: 10.1177/1087054713520617. PMID: 24505061.
- Catalá-López F, Hutton B, Núñez-Beltrán A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12(7):e0180355. doi: 10.1371/journal.pone.0180355. PMID: 28700715.
- Joseph A, Ayyagari R, Xie M, et al. Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry. 2017 Aug;26(8):875-97. doi: 10.1007/s00787-017-0962-6. PMID: 28258319.
- Padilha S, Virtuoso S, Tonin FS, et al. Efficacy and safety of drugs for attention deficit hyperactivity disorder in children and adolescents: a network meta-analysis. Eur Child Adolesc Psychiatry. 2018 Oct;27(10):1335-45. doi: 10.1007/s00787-018-1125-0. PMID: 29460165.
- Anand S, Tong H, Besag FMC, et al. Safety, Tolerability and Efficacy of Drugs for Treating Behavioural Insomnia in Children with Attention-Deficit/Hyperactivity Disorder: A Systematic Review with Methodological Quality Assessment. Paediatr Drugs. 2017 Jun;19(3):235-50. doi: 10.1007/s40272-017-0224-6. PMID: 28391425.
- Tsujii N, Usami M, Naya N, et al. Efficacy and Safety of Medication for Attention-Deficit Hyperactivity Disorder in Children and Adolescents with Common Comorbidities: A Systematic Review. Neurol Ther. 2021 Jun 4. doi: 10.1007/s40120-021-00249-0. PMID: 34089145.
- Maglione M, Das L, Motala A, et al. Surveillance Assessment on CER 44: Attention Deficit Hyperactivity Disorder (ADHD): Effectiveness of Treatment in At-Risk Preschoolers; Long-term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment (CER 44) Agency for Healthcare Research and Quality. Rockville, MD: July 2012 2012.
- Gidengil C, Goetz MB, Maglione M, et al. Safety of Vaccines Used for Routine Immunization in the United States: An Update. Comparative Effectiveness Review No. 244. (Prepared by the Southern California Evidence-based Practice Center under Contract No. 290-2015-00010-I.) AHRQ Publication No. 21-EHC024. . Rockville, MD: Agency for Healthcare Research and Quality (AHRQ); 2021.
- Hempel S, Graham GD, Fu N, et al. A systematic review of modifiable risk factors in the progression of multiple sclerosis. Mult Scler. 2017 Apr;23(4):525-33. doi: 10.1177/1352458517690270. PMID: 28151053.
- Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011 Apr(200):1-645. PMID: 23126627.
- Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012 May 9;307(18):1959-69. doi: 10.1001/jama.2012.3507. PMID: 22570464.
- Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. doi: 10.1136/bmj.l4898. PMID: 31462531.
- University of Bristol. QUADAS-2. Accessed on February 10, 2021.
- Rover C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015 Nov 14;15:99. doi: 10.1186/s12874-015-0091-1. PMID: 26573817.
- Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Content last reviewed March 2021. Effective Health Care Program Agency for Healthcare Research and Quality. Rockville, MD: 2021
None
None
The Agency for Healthcare Research and Quality (AHRQ) posted the Key Questions for the original, 2018 review on the AHRQ Effective Health Care Website for public comment in August 2021. The Evidence-based Practice Center (EPC) refined and finalized the Key Questions after review of the public comments, and input from Key Informants and the Technical Expert Panel (TEP). This input was intended to ensure that the key questions are specific and relevant.
Key Informants are the end users of research, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making health care decisions. Within the EPC program, the Key Informant role is to provide input into identifying the Key Questions for research that will inform healthcare decisions. The EPC solicited input from Key Informants on questions from the 2018 systematic review. Key Informants are not involved in analyzing the evidence or writing the report and have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism.
Key Informants must disclose any financial conflicts of interest greater than $5,000 and any other relevant business or professional conflicts of interest. Because of their role as end-users, individuals are invited to serve as Key Informants and those who present with potential conflicts may be retained. The AHRQ Task Order Officer (TOO) and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
Technical Experts constitute a multi-disciplinary group of clinical, content, and methodological experts who provide input in defining populations, interventions, comparisons, or outcomes and identify particular studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicting opinions are common and perceived as healthy scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, design, and methodological approaches do not necessarily represent the views of individual technical and content experts. Technical Experts provide information to the EPC to identify literature search strategies and suggest approaches to specific issues as requested by the EPC. Technical Experts do not do analysis of any kind nor do they contribute to the writing of the report. They have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism.
Technical Experts must disclose any financial conflicts of interest greater than $5,000 and any other relevant business or professional conflicts of interest. Because of their unique clinical or content expertise, individuals are invited to serve as Technical Experts and those who present with potential conflicts may be retained. The AHRQ TOO and the EPC work to balance, manage, or mitigate any potential conflicts of interest identified.
A TEP for this update review was convened. TEP input was sought to hone and re-affirm methods in the draft protocol, including perspectives on proposed KQ and PICOTS changes, approaches to new data integration, managing challenges and reporting to enhance usability and inform meaningful presentation of the report.
Peer reviewers are invited to provide written comments on the draft report based on their clinical, content, or methodological expertise. The EPC considers all peer review comments on the draft report in preparation of the final report. Peer reviewers do not participate in writing or editing of the final report or other products. The final report does not necessarily represent the views of individual reviewers. The EPC will complete a disposition of all peer review comments. The disposition of comments for systematic reviews and technical briefs will be published three months after the publication of the evidence report.
Potential Peer Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Invited Peer Reviewers may not have any financial conflict of interest greater than $5,000. Peer reviewers who disclose potential business or professional conflicts of interest may submit comments on draft reports through the public comment mechanism.
EPC core team members must disclose any financial conflicts of interest greater than $1,000 and any other relevant business or professional conflicts of interest. Related financial conflicts of interest that cumulatively total greater than $1,000 will usually disqualify EPC core team investigators.
This project was commissioned and funded by the Patient-Centered Outcomes Research Institute (PCORI) and executed under Contract No. 290-2015-00009-I from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The AHRQ Task Order Officer reviewed contract deliverables for adherence to contract requirements and quality. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by PCORI, the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
This protocol will be registered in the international prospective register of systematic reviews (PROSPERO).
Appendix A. Search strategies
Database: PubMed
Search terms
1 "Attention Deficit Disorder with Hyperactivity"[Mesh] OR "attention deficit hyperactivity disorder"[tiab] OR "ADHD"[tiab] OR "attention deficit disorder"[tiab]
2 "Pediatrics"[Mesh] OR "Adolescent"[Mesh] OR "Infant"[Mesh] OR "Child"[Mesh] OR child[tiab] OR children[tiab] OR infant[tiab] OR infants[tiab] OR preschool[tiab] OR preschooler[tiab] OR pediatric [tiab] OR teenager[tiab] OR teenagers[tiab] OR teenaged[tiab] OR teen[tiab] OR teens[tiab] OR adolescent[tiab] OR adolescents[tiab] OR adolescence[tiab] OR youth[tiab] OR paediatric[tiab] OR youths[tiab]
3 "Attention Deficit and Disruptive Behavior Disorders/diagnosis"[Majr] OR mass screening[mesh] OR questionnaires[mesh] OR Interviews as Topic[Mesh] OR Psychometrics[Mesh] OR Psychiatric Status Rating Scales[Mesh] OR diagnosis[mesh:noexp] OR "Diagnostic Techniques and Procedures"[Mesh] OR "Diagnostic and Statistical Manual of Mental Disorders"[Mesh] OR "Referral and Consultation"[Mesh] OR questionnaire[tiab] OR questionnaires[tiab] OR screening[tiab] OR screen[tiab] OR scale[tiab] OR instrument[tiab] OR instruments[tiab] OR interview[tiab] OR interviews[tiab] OR DSM[tiab] OR diagnosis[tiab] OR diagnostic[tiab] OR diagnosed[tiab] OR (Vanderbilt[tiab] AND scale[tiab]) OR conners[tiab] OR cprs[tiab] OR ctrs[tiab] OR crs[tiab] OR "snap-IV"[tiab] OR "snap-4"[tiab] OR "basc-2"[tiab] OR "behavioral assessment system for children"[tiab] OR dbdrs[tiab] OR "disruptive behavior disorder rating scale"[tiab] OR adhd-rs[tiab] OR "adhd rating scale"[tiab] OR ksads[tiab] OR k-sads[tiab] OR kiddie-sads[tiab] OR DISC[tiab] OR "diagnostic interview schedule for children"[tiab] OR "mini-kid"[tiab] OR "iva-2"[tiab] OR tova[tiab] OR "test of variables of attention"[tiab] OR neba[tiab] OR test[tiab] OR tests[tiab] OR testing[tiab] OR “Attention Deficit Disorder with Hyperactivity/diagnostic imaging”[Majr]
4 "Sensitivity and Specificity"[Mesh] OR "Diagnostic Errors"[Mesh] OR sensitivity[tiab] OR specificity[tiab] OR accuracy[tiab] OR accurate[tiab] OR accurately[tiab] OR misdiagnos*[tiab] OR (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tiab] OR "clinical trials"[tiab] OR "evaluation studies"[pt] OR "evaluation studies as topic"[MeSH] OR "evaluation study"[tiab] OR evaluation studies[tiab] OR "intervention studies"[MeSH] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "cohort studies"[MeSH] OR cohort[tiab] OR "longitudinal studies"[MeSH] OR "longitudinal"[tiab] OR longitudinally[tiab] OR "prospective"[tiab] OR prospectively[tiab] OR "comparative study"[pt] OR "comparative study"[tiab] OR systematic[sb] OR "ROC Curve"[tiab] OR "positive predictive value"[tiab] OR "negative predictive value"[tiab] OR "false positive"[tiab] OR "false negative"[tiab] OR "likelihood ratio"[tiab]) NOT (Editorial[ptyp] OR Letter[pt] OR Case Reports[pt] OR Comment[pt] address[pt] OR “autobiography”[pt] OR “bibliography”[pt] OR “biography”[pt] OR "case report"[tw] OR “case reports”[tw] OR “case series”[tw] OR "comment on"[All Fields] OR congress[pt] OR “dictionary”[pt] OR “directory”[pt] OR “festschrift”[pt] OR “historical article”[pt] OR lecture[pt] OR "legal case"[pt] OR “legislation”[pt] OR “news”[pt] OR “newspaper article”[pt] OR “patient education handout”[pt] OR “periodical index”[pt]) NOT (animals[mh] NOT humans[mh]) AND English[la]
#1 AND #2 AND #3 AND #4
Search terms
1 "Attention Deficit Disorder with Hyperactivity"[Mesh] OR "attention deficit hyperactivity disorder"[tiab] OR "ADHD"[tiab] OR "attention deficit disorder"[tiab]
2 "Pediatrics"[Mesh] OR "Adolescent"[Mesh] OR "Infant"[Mesh] OR "Child"[Mesh] OR child[tiab] OR children[tiab] OR infant[tiab] OR infants[tiab] OR preschool[tiab] OR preschooler[tiab] OR pediatric[tiab] OR teenager[tiab] OR teenagers[tiab] OR teenaged[tiab] OR teen[tiab] OR teens[tiab] OR adolescent[tiab] OR adolescents[tiab] OR adolescence[tiab] OR youth[tiab]
3 #1 AND #2
4 Azstarys OR Cotempla XR-ODT OR Desoxyn OR "Alpha agonist" OR "Attention Deficit Disorder with Hyperactivity/drug therapy"[Majr] OR "Central Nervous System Stimulants"[MeSH] OR "Methylphenidate"[MeSH] OR "Dexmethylphenidate"[MeSH] OR "Dextroamphetamine"[MeSH] OR "Adderall"[Supplementary Concept] OR "lisdexamfetamine dimesylate"[Supplementary Concept] OR "Amphetamine"[MeSH] OR "Guanfacine"[MeSH] OR "Sympatholytics"[MeSH] OR "Clonidine"[MeSH] OR "Adrenergic Uptake Inhibitors"[MeSH] OR "Adrenergic Uptake Inhibitors"[Pharmacological Action] OR "Receptors, Adrenergic, alpha-2"[MeSH] OR "Adrenergic alpha-Agonists"[Mesh] OR "Adrenergic alpha-2 Receptor Agonists"[Mesh] OR "atomoxetine"[Supplementary Concept] OR "Antidepressive Agents, Tricyclic"[MeSH] OR "Desipramine"[MeSH] OR "Dopamine Uptake Inhibitors"[MeSH] OR "Sympathomimetics"[MeSH] OR "modafinil"[Supplementary Concept] OR "Serotonin Uptake Inhibitors"[MeSH] OR "Serotonin Uptake Inhibitors"[Pharmacological Action] OR "duloxetine" [Supplementary Concept] OR "Monoamine Oxidase Inhibitors"[MeSH] OR "Monoamine Oxidase"[MeSH] OR "Selegiline"[MeSH] OR "Bupropion"[MeSH] OR "armodafinil" [Supplementary Concept] OR "venlafaxine"[Supplementary Concept] OR "Receptors, N-Methyl-D-Aspartate"[MeSH] OR "Memantine"[MeSH] OR "Amantadine"[MeSH] OR "duloxetine"[Supplementary Concept] OR "Central Nervous System Stimulants" [Pharmacological Action] OR "Adrenergic alpha-2 Receptor Agonists" [Pharmacological Action] OR "Antidepressive Agents, Tricyclic" [Pharmacological Action] OR "Dopamine Uptake Inhibitors" [Pharmacological Action] OR "Monoamine Oxidase Inhibitors" [Pharmacological Action] OR "Central Nervous System Stimulants"[tiab] OR "psychostimulant"[tiab] OR "Methylphenidate"[tiab] OR "Methylphenidate Hydrochloride"[tiab] OR "Aptensio"[tiab] OR "Concerta"[tiab] OR "Ritalin"[tiab] OR "Ritalin LA"[tiab] OR "Medikinet"[tiab] OR "Equasym"[tiab] OR "Quillivant"[tiab] OR "Metadate"[tiab] OR "Daytrana"[tiab] OR "Dexmethylphenidate"[tiab] OR "Dexmethylphenidate Hydrochloride"[tiab] OR "Focalin"[tiab] OR "Dextroamphetamine"[tiab] OR "Dexedrine"[tiab] OR "Dextrostat"[tiab] OR "ProCentra"[tiab] OR "Zenzedi"[tiab] OR "mixed amphetamine salts"[tiab] OR "Adderall" [tiab] OR "lisdexamfetamine"[tiab] OR "lisdexamfetamine dimesylate"[tiab] OR "Vyvanse"[tiab] OR "Venvanse"[tiab] OR "Elvanse"[tiab] OR "Tyvense"[tiab] OR "Dyanavel"[tiab] OR "Evekeo"[tiab] OR "Guanfacine"[tiab] OR "Sympatholytics"[tiab] OR "Central alpha-2 Adrenergic Agonist"[tiab] OR "Clonidine"[tiab] OR "Intuniv"[tiab] OR "Estulic"[tiab] OR "Tenex"[tiab] OR "Catapres"[tiab] OR "Clophelin"[tiab] OR "Kapvay"[tiab] OR "Nexiclon"[tiab] OR "Duraclon"[tiab] OR "Norepinephrine Reuptake Inhibitors"[tiab] OR "Selective Norepinephrine Reuptake Inhibitors"[tiab] OR "Adrenergic Uptake Inhibitors"[tiab] OR "atomoxetine"[tiab] OR "Strattera"[tiab] OR "Tricyclic antidepressants"[tiab] OR "Desipramine"[tiab] OR "Norpramin"[tiab] OR "Nortriptyline"[tiab] OR "Pamelor"[tiab] OR "Dopamine Reuptake Inhibitors"[tiab] OR "modafinil"[tiab] OR "Provigil"[tiab] OR "Armodafinil"[tiab] OR "Norepinephrine-dopamine Reuptake Inhibitors"[tiab] OR "Bupropion"[tiab] OR "Wellbutrin"[tiab] OR "Forfivo"[tiab] OR "Cymbalta"[tiab] OR "venlafaxine"[tiab] OR "reboxetine"[tiab] OR "Monoamine Oxidase Type B inhibitors"[tiab] OR "Selegiline"[tiab] OR "Eldepryl"[tiab] OR "Zelapar"[tiab] OR "NMDA receptors"[tiab] OR "N-Methyl-D-aspartate receptor Antagonists"[tiab] OR "Amantadine"[tiab] OR "Memantine"[tiab] OR "Pertofrane"[tiab] OR "Nuvigil"[tiab] OR "Cymbalta"[tiab] OR "duloxetine"[tiab] OR "Effexor"[tiab] OR "Eldepryl"[tiab] OR "Emsam"[tiab] OR "Trevilor"[tiab] OR "Symmetrel"[tiab] OR "Namenda"[tiab] OR "Zelapar"[tiab]
5 Monarch external Trigeminal Nerve Stimulation OR eTNS OR "EndeavorRx" OR ((classroom[Title/Abstract] OR school OR schools) AND (behavior intervention[Title/Abstract] OR behavior interventions[Title/Abstract])) OR "peer intervention" OR (("organization skills"[Title/Abstract]) AND (training[Title/Abstract] OR intervention[Title/Abstract])) OR "Attention Deficit Disorder with Hyperactivity/diet therapy"[Majr] OR "Attention Deficit Disorder with Hyperactivity/rehabilitation"[Majr] OR "Psychotherapy"[MeSH] OR "Behavior Therapy"[MeSH] OR "Parent-Child Relations"[MeSH] OR "Play Therapy"[MeSH] OR "Cognitive Therapy"[MeSH] OR "Time Management"[MeSH] OR "Computer-Assisted Instruction"[MeSH] OR "Diet Therapy"[MeSH] OR "Fatty Acids, Omega-3/therapeutic use"[MeSH] OR "Vitamins/administration and dosage"[Mesh] OR "Vitamins/therapeutic use"[MeSH] OR "Food Additives/adverse effects"[MeSH] OR "Probiotics/therapeutic use"[MeSH] OR "Acupuncture Therapy"[MeSH] OR "Remedial Teaching"[MeSH] OR "Early Intervention (Education)"[MeSH] OR "Complementary Therapies"[MeSH] OR "Combined Modality Therapy"[MeSH] OR "psychosocial therapy"[tiab] OR "psychosocial intervention"[tiab] OR "psychosocial interventions"[tiab] OR "psychosocial approach"[tiab] OR "psychosocial approaches"[tiab] OR "psychosocial treatment"[tiab] OR "psychosocial support"[tiab] OR "psychoeducation"[tiab] OR "nonpharmacologic therapy"[tiab] OR "nondrug therapy"[tiab] OR "non-drug therapy"[tiab] OR "Play Therapy"[tiab] OR "cognitive behavioral therapy"[tiab] OR "cognitive behavior therapy"[tiab] OR "cognitive behavioural therapy"[tiab] OR "cognitive behaviour therapy"[tiab] OR Mindfulness[tiab] OR complementary[tiab] OR "alternative medicine"[tiab] OR "alternative therapy"[tiab] OR "alternative therapies"[tiab] OR "Interpersonal skills training"[tiab] OR "Parent-Child Interaction Therapy"[tiab] OR "parent training"[tiab] OR "parent engagement"[tiab] OR "parent management"[tiab] OR "parenting skills"[tiab] OR "parenting intervention"[tiab] OR "parenting interventions"[tiab] OR "Barkley's defiant child"[tiab] OR "Teacher-Child Interaction Training"[tiab] OR "Incredible Years"[tiab] OR "New Forest Parenting"[tiab] OR "Triple P"[tiab] OR "Helping the Noncompliant Child"[tiab] OR "child life and attention skills"[tiab] OR "clas"[tiab] OR PCIT[tiab] OR "parent child interaction therapy"[tiab] OR "Summer Treatment Program"[tiab] OR "Daily Report Card"[tiab] OR "organization skills"[tiab] OR "organizational skills"[tiab] OR "time management"[tiab] OR "homework intervention"[tiab] OR braintrain[tiab] OR "memory training"[tiab] OR "Captain's log mindpower builder"[tiab] OR "memory gyms"[tiab] OR "attention gym"[tiab] OR "smartdriver plus"[tiab] OR "smartmind pro"[tiab] OR "RoboMemo"[tiab] OR "play attention"[tiab] OR metronome[tiab] OR brainmaster[tiab] OR mindmed[tiab] OR "attention lab"[tiab] OR (activate[tiab] AND c8[tiab]) OR "attention training"[tiab] OR "CogniPlus"[tiab] OR cogmed[tiab] OR "working memory training"[tiab] OR biofeedback[tiab] OR neurofeedback[tiab] OR neuroagility[tiab] OR neuroptimal[tiab] OR acupuncture[tiab] OR "vision training"[tiab] OR "visual training"[tiab] OR "vision therapy"[tiab] OR "education intervention"[tiab] OR "cognitive remediation"[tiab] OR neurotherapy[tiab] OR "elimination diet"[tiab] OR "diet therapy"[tiab] OR (("low carb" OR "low carbohydrate" OR "low carbohydrates"[tiab] OR "gluten free") AND diet[tiab]) OR "feingold diet"[tiab] OR "red dye"[tiab] OR ((vitamin[tiab] OR vitamins[tiab]) AND (supplement[tiab] OR supplements[tiab])) OR "herbal supplement"[tiab] OR "herbal supplements"[tiab] OR probiotics[tiab] OR "omega 3"[tiab] OR "slow cortical potentials"[tiab] OR "few foods diet"[tiab] OR "oligoantigenic diet"[tiab] OR "restriction diet"[tiab] OR "food intolerance"[tiab] OR "food allergy"[tiab] OR "food allergies"[tiab] OR "food sensitivity"[tiab] OR "food sensitivities"[tiab] OR "multimodal treatment"[tiab] OR homeopathy[tiab] OR homeopathic[tiab] OR chiropractic[tiab] OR chiropractor[tiab]
6 #4 OR #5
7 #3 AND #6
8 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tiab] OR "clinical trials"[tiab] OR "evaluation studies"[pt] OR "evaluation studies as topic"[MeSH] OR "evaluation study"[tiab] OR "evaluation studies"[tiab] OR "intervention studies"[MeSH] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "case-control studies"[MeSH] OR "case-control"[tiab] OR "cohort studies"[MeSH] OR cohort[tiab] OR "longitudinal"[tiab] OR longitudinally[tiab] OR "prospective"[tiab] OR prospectively[tiab] OR "retrospective"[tiab] OR "comparative study"[pt] OR "comparative study"[tiab] OR systematic[sb] OR "meta-analysis"[pt] OR "meta-analysis as topic"[MeSH] OR "meta-analysis"[tiab] OR "metaanalyses"[tiab]) NOT (Editorial[ptyp] OR Letter[pt] OR Case Reports[pt] OR Comment[pt]) NOT (animals[mh] NOT humans[mh]) AND English[la]
9 #7 AND #8
Search terms
1 "Attention Deficit Disorder with Hyperactivity"[Mesh] OR "attention deficit hyperactivity disorder"[tiab] OR "ADHD"[tiab] OR "attention deficit disorder"[tiab]
2 "Pediatrics"[Mesh] OR "Adolescent"[Mesh] OR "Infant"[Mesh] OR "Child"[Mesh] OR child[tiab] OR children[tiab] OR infant[tiab] OR infants[tiab] OR preschool[tiab] OR preschooler[tiab] OR pediatric[tiab] OR teenager[tiab] OR teenagers[tiab] OR teenaged[tiab] OR teen[tiab] OR teens[tiab] OR adolescent[tiab] OR adolescents[tiab] OR adolescence[tiab] OR youth[tiab]
3 monitor[tiab] OR monitored[tiab] OR monitoring[tiab] OR "follow up"[tiab] OR "followed up"[tiab] OR visit[tiab] OR visits[tiab] OR session[tiab] OR sessions[tiab] OR appointment[tiab] OR appointments[tiab]
4 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[pt] OR "clinical trial"[tiab] OR "clinical trials"[tiab] OR "evaluation studies"[pt] OR "evaluation studies as topic"[MeSH] OR "evaluation study"[tiab] OR "evaluation studies"[tiab] OR "intervention studies"[MeSH] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "case-control studies"[MeSH] OR "case-control"[tiab] OR "cohort studies"[MeSH] OR cohort[tiab] OR "longitudinal"[tiab] OR longitudinally[tiab] OR "prospective"[tiab] OR prospectively[tiab] OR "retrospective"[tiab] OR "comparative study"[pt] OR "comparative study"[tiab] OR systematic[sb] OR "meta-analysis"[pt] OR "meta-analysis as topic"[MeSH]) NOT (Editorial[ptyp] OR Letter[pt] OR Case Reports[pt] OR Comment[pt]) NOT (animals[mh] NOT humans[mh]) AND English[la]
5 #1 AND #2 AND #3 AND #4




