On June 28, 2017 and October 26, 2017, amendments were made to this protocol. To view the amendments, go to Summary of Protocol Amendments.
I. Background and Objectives for the Systematic Review
This systematic review is an update of an earlier report published in 2013 which evaluated questions related to stroke prevention in patients with atrial fibrillation (AF) and atrial flutter.1 The original review found that CHADS2 and CHADS2-VASc scores have the best discrimination ability for stroke events in patients with AF, whereas HAS-BLED provides the best discrimination of bleeding risk. There was insufficient evidence on imaging tools such as transthoracic echo (TTE), transesophageal echo (TEE), CT scans, or cardiac magnetic resonance imaging (MRI). Newer anticoagulants (direct oral anticoagulants [DOACs]) resulted in reduced stroke and bleeding events when compared with warfarin, and apixaban showed better efficacy and similar safety and in patients who are not candidates for warfarin. Given the uncertainties which remained within the limitations of the available evidence, and the new data which have emerged since that report, an update of the systematic review was commissioned.
Epidemiology of Atrial Fibrillation
Atrial fibrillation (AF) is an irregular supraventricular tachyarrhythmia (any tachycardic rhythm originating above the ventricular tissue) and is characterized by uncoordinated atrial activation with consequent deterioration of mechanical function.2 Atrial flutter is a common abnormal heart rhythm, similar to AF. Both conditions are types of supraventricular tachycardia. In atrial flutter, the upper chambers of the heart beat too fast, which results in atrial muscle contractions that are faster than and out of sync with the lower chambers. Within this systematic review, we will use AF to include patients with either atrial fibrillation and atrial flutter.
AF is the most common cardiac arrhythmia seen in clinical practice, accounting for approximately one-third of hospitalizations for cardiac rhythm disturbances. The estimated prevalence of AF is 0.4 percent to 1 percent in the general population,3,4 occurring in about 2.2 million people in the United States. The prevalence increases to about 6 percent in people aged 65 or older and to 10 percent in people aged 80 or older.5 Management of AF involves three distinct areas, namely, rate control, rhythm control, and prevention of thromboembolic events. This review will focus on prevention of thromboembolic events.
Atrial Fibrillation and Stroke
Although generally not as immediately life-threatening as ventricular arrhythmias, AF is associated with significant morbidity and mortality. Patients with AF have increased risk of embolic stroke, heart failure, and cognitive impairment; reduced quality of life; and higher overall mortality.6-8 Patients with AF have a five-fold increased risk of stroke, and it is estimated that up to 25 percent of all strokes in the elderly are a consequence of AF.5 Furthermore, AF-related strokes are more severe than other types of stroke, with AF patients being twice as likely to become bedridden than patients with stroke from other etiologies and more likely to die from the stroke.9-11 Consistent with the nature of these events, AF-related stroke constitutes a significant economic burden, costing Medicare approximately $8 billion annually.12
Risk Stratification and Stroke Prevention
Risk Stratification
The rate of ischemic stroke among patients with nonvalvular AF averages 5 percent per year, 2 to 7 times that of the general population.9 The risk of stroke increases from 1.5 percent for patients with AF who are 50 to 59 years old to 23 percent for those who are 80 to 89 years old.10 Congestive heart failure, Hypertension, older Age, Diabetes mellitus, prior Stroke or transient ischemic attack (TIA), Vascular disease, and female Sex are considered independent risk factors for stroke and several of these factors are associated with AF. These risk factors are the elements that form the CHADS2 and CHADS2-VASc scores.13,14 The CHADS2 score ranges from 0 to 6, with increasing scores corresponding to increasing stroke risk, and is easy to calculate and apply in clinical practice. The adjusted annual rates of stroke vary from 1.9 percent in patients with a CHADS2 score of 0 to 18.2 percent in patients with a CHADS2 score of 6.13 Similarly, the CHADS2-VASc score ranges from 0 to 9, with increasing scores corresponding to increasing stroke risk, and is easy to calculate and apply in clinical practice.2 The adjusted annual rates of stroke vary from 1.3 percent in patients with a CHADS2-VASc score of 1 to 15.2 percent in patients with a CHADS2-VASc score of 9.15
A number of studies have examined the appropriate populations and appropriate therapies for adequate stroke prophylaxis in AF. Despite existing risk stratification tools with overlapping characteristics, the major risk factors for ischemic stroke and systemic embolism in patients with nonvalvular AF are congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or TIA, vascular disease and female sex. As mentioned previously, these factors comprise the CHADS2-VASc score. The 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation recommends the use the CHADS2-VASc score to estimate the stroke risk, and oral anticoagulation is indicated for patients with a score ≥2, and it should be considered for patients with a score of 1 (i.e., with one risk factor16). The HAS-BLED (H=hypertension, A=abnormal renal/liver function, S=stroke, B=bleeding history or predisposition, L=labile INR, E=elderly (> 65), D=drugs/alcohol concomitantly) score is used to identify potentially reversible factors that contribute to bleeding, in order to avoid and correct them when possible. Scores on this scale range from 0 to 9. A score ≥3 indicates a high risk of bleeding with oral anticoagulation and/or antiplatelets agents such as aspirin. Based on the original systematic review, however, the strength of evidence was low for the CHADS2-VASc score and moderate for the HAS-BLED score. Following the initial evidence review, several evidence gaps remained including how best to predict the overall clinical risk of patients (combining both their risk of stroke and their risk of bleeding) and how to increase the dissemination of point-of-care tools to improve risk assessment and guide treatment choices for clinicians.
Therapeutic Options for Stroke Prevention in AF
Much of the focus of AF management has been on treatment strategies for stroke prevention. Antithrombotic therapies are the mainstays used to prevent thromboembolic events in patients with AF. Systemic anticoagulation has been shown to reduce the risk of stroke by two-thirds. Unfortunately, two critical issues regarding stroke prevention in AF remain: (1) despite existing evidence, only a minority of patients who have AF and are at risk for stroke receive optimal treatment for thromboembolic prevention,17,18 and (2) patients with AF on stroke prophylaxis with warfarin still have higher rates of stroke than non-AF patients16, suggesting that gaps still exist in our understanding of risk stratification and treatment. With the introduction of DOACs for stroke prevention, providers, and patients have wider choices available for treatment. Accordingly, identifying high-risk patients and choosing the optimal treatment has become even more complex.
Table 1 provides an overview of the therapeutic options currently considered for stroke prevention for patients with atrial fibrillation. Following recent recommendations from the European Society of Cardiology19 on the management of AF, antiplatelets are no longer recommended for stroke prevention in AF. Because the ACC/AHA/HRS Guidelines have not yet been updated with a similar recommendation16, we include antiplatelets as a comparator of interest but do not include it within the Table below of primary therapeutic options.
| Treatment | Description |
|---|---|
| Vitamin K antagonists (VKA) | VKAs such as warfarin, have been the standard-of-care for stroke prevention in patients with AF for decades. However, it is often difficult to achieve and maintain the international normalized ratio (INR), a measure of anticoagulation, within a therapeutic range (2.0–3.0), and multiple food and drug interactions make the management of VKAs very difficult. In addition, the need to monitor the INR on a regular basis can discourage some patients from taking VKAs. These important challenges associated with VKA treatment have ignited the interest in developing novel therapeutic options, with better efficacy and safety profiles. |
| Direct oral anticoagulants (DOACs) | Currently, there are four DOACs approved for stroke prevention in patients with non-valvular AF: dabigatran (thrombin inhibitor), apixaban, rivaroxaban, and edoxaban (all factor Xa inhibitors). These agents have been studied in large randomized trials. These studies showed that DOACs are at least as efficacious and, except for high dose dabigatran, significantly safer than warfarin, with a clear benefit in reducing the occurrence of intracranial hemorrhage.20-23 These drugs are easier to use as there is no need for periodic laboratory monitoring. With the availability of these drugs for clinical use, additional knowledge is needed to help inform decision-making related to whether these medications are safe and effective in patient populations not included or not well-represented in clinical trials and to better understand the relative risks and benefits of these drugs based on individual patient characteristics. |
| Procedural interventions | Procedural interventions for stroke prophylaxis have emerged and are growing in their use. For example, left atrial appendage (LAA) occlusive devices are an alternative treatment strategy used to prevent blood clot formation in patients with AF. Although evidence is sparse, for patients with AF who are elderly (at high risk for falls), have a prior bleeding history, are pregnant, and/or noncompliant, LAA occlusion may be a better stroke prevention strategy. |
There are several areas of insufficient evidence and uncertainty within the field of stroke prevention in patients with AF:
- The comparative diagnostic accuracy and impact on clinical decision-making of available clinical and imaging tools for predicting thromboembolic and bleeding risk in patients with AF are uncertain
- There is a lack of information to guide decisions regarding the best specific anticoagulant (versus warfarin) for a given patient.
- The safety and effectiveness of DOACs is unclear in patients not included or not well-represented in randomized clinical trials (e.g., patients with moderate to severe chronic kidney disease (CKD) [eGFR<60], valvular heart disease, extremes of BMI, older age, women, multiple comorbidities, and a history of bleeding or frequent falls).
- The relative safety and effectiveness of DOACs as compared to left atrial appendage (LAA) occlusion devices is uncertain.
II. The Key Questions
The key questions (KQs) for this systematic review update derive from the original review and have been updated based on stakeholder feedback obtained by PCORI. Specifically our key questions are:
- In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic and patient outcome efficacy) of available clinical and imaging tools and associated risk factors for predicting thromboembolic risk?
- In patients with nonvalvular atrial fibrillation, what are the comparative diagnostic accuracy and impact on clinical decisionmaking (diagnostic thinking, therapeutic, and patient outcome efficacy) of clinical tools and associated risk factors for predicting bleeding events?
- What are the comparative safety and effectiveness of specific anticoagulation therapies, antiplatelet therapies, and procedural interventions for preventing thromboembolic events:
- In patients with nonvalvular atrial fibrillation?
- In specific subpopulations of patients with nonvalvular atrial fibrillation
Contextual Question (CQ)
Contextual questions are not systematically reviewed and use a "best evidence" approach. Information about the contextual questions may be included as part of the introduction or discussion section and related as appropriate to the SR.
CQ
What are currently available shared decision-making tools for patient and provider use for stroke prophylaxis in atrial fibrillation, and what are their relative strengths and weaknesses?
III. Analytic Framework
Provisional analytic framework for the prevention of stroke in patients with atrial fibrillation
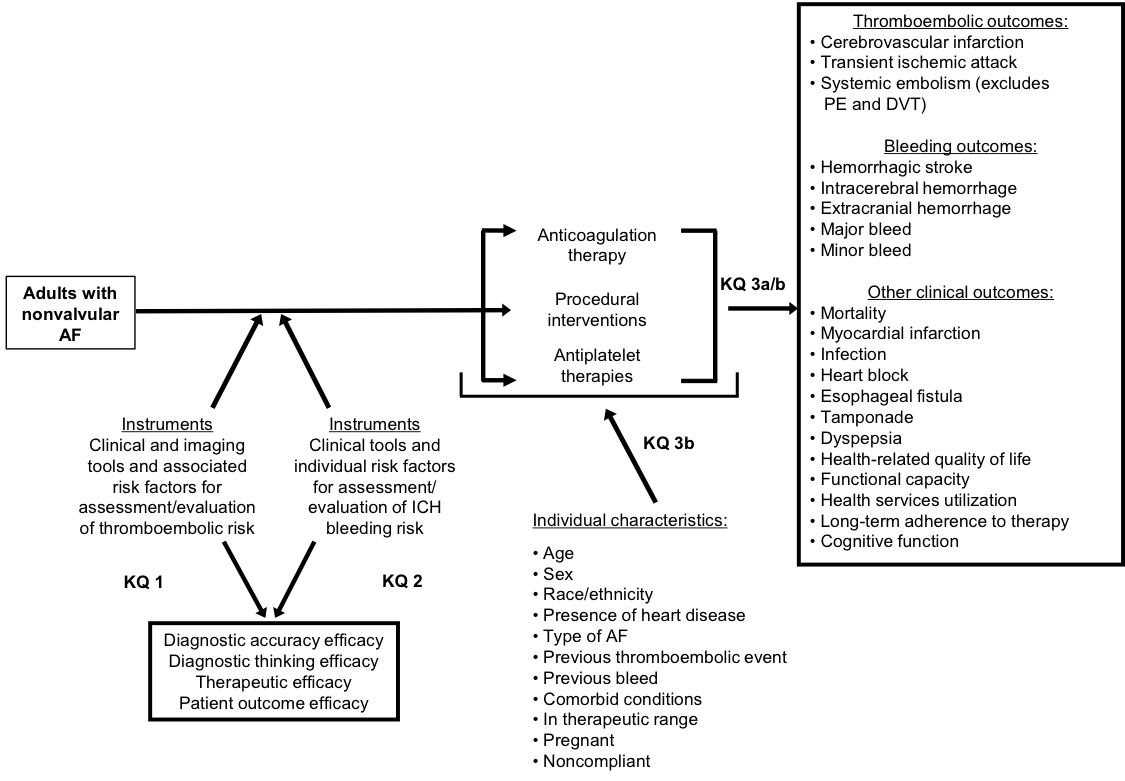
Abbreviations: AF = atrial fibrillation; ICH = intracerebral hemorrhage; KQ = key question; PE = pulmonary embolism; DVT = deep vein thrombosis
IV. Methods
In developing this comprehensive review, we will apply the rules of evidence and evaluation of strength of evidence recommended by the Agency for Healthcare Research and Quality (AHRQ)'s EPC Program in its Methods Guide for Effectiveness and Comparative Effectiveness Reviews (hereafter referred to as the Methods Guide)24 and the Methods Guide for Medical Test Reviews.25 We will follow the methodology recommended by the EPCs for literature search strategies, inclusion/exclusion of studies in our review, abstract screening, data abstraction and management, assessment of methodological quality of individual studies, data synthesis, and grading of evidence for each KQ.
A. Criteria for Inclusion/Exclusion of Studies in the Review
We will apply the inclusion and exclusion criteria described in Table 2 below to studies identified by our literature search.
| PICOTS Element | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| aDue to: (1) the high volume of literature available in English language publications, (2) the focus of our review on applicability to populations in the United States, and (3) the scope of our KQs, it is the opinion of the investigators that the resources required to translate non-English articles would not be justified by the low potential likelihood of identifying relevant data unavailable from English-language sources. bThere are different classification systems for bleeding (e.g. International Society on Thrombosis and Haemostasis (ISTH), Global Utilization Of Streptokinase And Tpa For Occluded Arteries (GUSTO), Thrombolysis In Myocardial Infarction (TIMI)). Different studies have used different systems of classification so we will report data based on the studies classification and we will capture what system they used for classification and incorporate this information in to any potential quantitative synthesis of the data. We do not expect studies to provide enough granular data to classify the events ourselves. Abbreviations: ABC = age, biomarkers, clinical history; AF = atrial fibrillation; ATRIA = age, female, diabetes, congestive heart failure, hypertension, proteinuria, eGFR < 45 or ESRD; CHADS2 = congestive heart failure, hypertension, age > 75, diabetes, stroke/TIA; CHADS2-VASc = congestive heart failure/left ventricular ejection fraction ≤ 40%, hypertension, age ≥ 75, diabetes, stroke/TIA/thromboembolism, vascular disease, age 65-74, sex; CT = computed tomography; DM = diabetes mellitus; DTI = direct thrombin inhibitor; DVT = deep vein thrombosis; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (> 65), drugs/alcohol concomitantly; HEMORR2HAGES = hepatic or renal disease, ethanol (alcohol) abuse, malignancy, older (> 75), reduced platelet count or function, rebleeding risk, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, stroke history; INR = international normalized ratio; KQ = key question; MRI = magnetic resonance imaging; PE = pulmonary embolism; PICOTS = Populations, Interventions, Comparators, Outcomes, Timing, Settings; PLAATO = Percutaneous Left Atrial Appendage Transcatheter Occlusion; RCT = randomized controlled trial; TIA = transient ischemic attack; VKA = Vitamin K antagonists. |
||
| Populations |
|
|
| Interventions | KQ 1: Clinical and imaging tools and associated risk factors for assessment/evaluation of thromboembolic risk:
|
None |
| Comparators |
|
For KQ 3, studies that did not include an active comparator |
| Outcomes |
|
Study does not include any outcomes of interest |
| Timing | Timing of follow-up not limited | None |
| Setting | Inpatient and outpatient | None |
| Study design |
|
|
| Publications |
|
|
B. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies to Answer the Key Questions
The evidence published from 2012 both represents the current standard of care for the population of interest in this review and allows this report to build on the previous systematic review published in 2013 (which had an electronic search date through August 2012). To identify relevant published literature, we will search PubMed®, Embase®, and the Cochrane Database of Systematic Reviews (CDSR), limiting the search to studies conducted in adults from August 1, 2011, to the present. These databases were selected based on the approaches utilized in the original systematic review, which the current systematic review is updating. Our proposed search strategy for PubMed is provided in Appendix A; this strategy will be adapted as appropriate for searching the other databases. Where possible, we will use existing validated search filters (such as the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE). An experienced search librarian will guide all searches. While the draft report is under peer review, we will update the search and include any eligible studies identified either during that search or through peer or public reviews in the final report. As described below, our findings will be combined with the findings from our original review for the specific key questions. The modifications to the key questions which differ between our original report's search and our update do not impact our search strategy but they do impact the outcomes of interest. We will therefore review the citations which were excluded from our previous systematic review because they did not include outcomes of interest (N=115)28 to determine which, if any, of these studies should now be included as part of our update.
We will supplement the electronic searches with a manual search of citations from a set of key primary and review articles identified during screening. The reference list for identified pivotal articles will be manually hand-searched and cross-referenced against our database, and additional relevant manuscripts will be retrieved. All citations will be imported into an electronic bibliographical database (EndNote® Version X7; Thomson Reuters, Philadelphia, PA).
We will use several approaches to identifying relevant gray literature, including requests to drug and device manufacturers and other stakeholders for scientific information packets. These requests will be coordinated by AHRQ's Scientific Resource Center. Additional grey literature will be solicited through a notice posted in the Federal Register and on the AHRQ Effective Health Care website. As a mechanism to ascertain publication bias in recent studies, we will search ClinicalTrials.gov to identify completed but unpublished studies (we will also explore the possibility of publication bias specifically in our quantitative synthesis of the included literature through meta-analysis techniques). We will also search ClinicalTrials.gov for relevant articles from completed studies.
For citations retrieved from MEDLINE, Embase, and the CDSR, two reviewers using prespecified inclusion/exclusion criteria will review titles and abstracts for potential relevance to the research questions. Inclusion at the title and abstract screening level will be liberal; if a single reviewer believes an article may contain relevant information, the article will move to the next level for further screening. Articles included by either reviewer will undergo full-text screening. At the full-text screening stage, two independent reviewers must agree on a final inclusion/exclusion decision. Disagreements that cannot be resolved by the two reviewers will be resolved by a third expert member of the team. Articles meeting eligibility criteria (see Table 2) will be included for data abstraction. At random intervals during screening, quality checks by senior team members will occur to ensure that screening and abstraction is consistent with inclusion/exclusion criteria and abstraction guidelines. We will make screening decisions and abstract data based on the published literature and available online appendices. Given the timeline and resources, we will not contact study authors for additional data. All results will be tracked using the DistillerSR data synthesis software program (Evidence Partners Inc., Manotick, ON, Canada).
To answer the CQ, we will search our included AF studies as well as reviews captured by our search that discuss currently available shared decision-making tools for stroke prophylaxis in atrial fibrillation. The CQ will be discussed within the context of the Discussion of the report.
C. Data Abstraction and Data Management
The research team will create data abstraction forms for the KQs that will be programmed in the DistillerSR software. Based on their clinical and methodological expertise, a pair of researchers will be assigned to abstract data from each of the eligible articles. One researcher will abstract the data, and the second will over-read the article and the accompanying abstraction to check for accuracy and completeness. Disagreements will be resolved by consensus or by obtaining a third reviewer's opinion if consensus cannot be reached. We will link studies to avoid duplication of patient cohorts.
We will design the data abstraction forms for this project to collect the data required to evaluate the specified eligibility criteria for inclusion in this review, as well as demographic and other data needed for determining outcomes (intermediate, final, and adverse events outcomes). We will pay particular attention to describing the details of the diagnostic tools (e.g., instrument version, administration mode), details of the treatment (e.g., dosing, co-interventions, methods of procedural therapies), patient characteristics (e.g., etiology of AF, history of prior bleed or stroke) and study design (e.g., RCT versus observational) that may be related to outcomes. In addition, we will describe comparators carefully, as treatment standards may have changed during the period covered by the review. The safety outcomes will be framed to help identify adverse events, including those from drug therapies and those resulting from procedural complications. Data necessary for assessing quality and applicability, as described in the Methods Guide,24 will also be abstracted. Before they are used, abstraction form templates will be pilot-tested with a sample of included articles to ensure that all relevant data elements are captured and that there is consistency and reproducibility between abstractors. Forms will be revised as necessary before full abstraction of all included articles. Final abstracted data will be uploaded to the Systematic Review Data Repository (SRDR) per EPC requirements.
D. Assessment of Methodological Quality of Individual Studies
We will assess methodological quality, or risk of bias, for each individual study using tools specific to the study's characteristics. For all studies, we will use the following strategy: (1) classify the study design, (2) apply predefined criteria for appraisal of quality, and (2) arrive at a summary judgement of the study's quality. For studies assessing diagnostic accuracy, we will use the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool and follow guidance for use of that tool to arrive at an overall judgement.22
For non-diagnostic studies, we will use the Cochrane Risk of Bias tool for randomized studies29,30 and the Risk Of Bias In Non-randomised Studies - of Interventions (ROBINS-I) tool for observational studies.31,32 Briefly, we will rate each study as being of good, fair, or poor quality based on its adherence to well-accepted standard methodologies. For each study, one investigator will assess methodological quality which will be reviewed by a second investigator; disagreements will be resolved by consensus or by a third investigator if agreement cannot be reached. Overall study quality will be assessed as follows:
- Good (low risk of bias). These studies had the least bias, and the results were considered valid. These studies adhered to the commonly held concepts of high quality, including the following: a clear description of the population, setting, approaches, and comparison groups; appropriate measurement of outcomes; appropriate statistical and analytical methods and reporting; no reporting errors; a low dropout rate; and clear reporting of dropouts.
- Fair. These studies were susceptible to some bias, but not enough to invalidate the results. They did not meet all the criteria required for a rating of good quality because they had some deficiencies, but no flaw was likely to cause major bias. The study may have been missing information, making it difficult to assess limitations and potential problems.
- Poor (high risk of bias). These studies had significant flaws that might have invalidated the results. They had serious errors in design, analysis, or reporting; large amounts of missing information; or discrepancies in reporting.
The quality assessment will be outcome-specific such that a given study that analyzes its primary outcome well but did an incomplete analysis of a secondary outcome would be assigned a different quality grade for each of the two outcomes. We will apply this outcome-specific quality assessment to groups of outcomes that have lower risk of detection bias (e.g., mortality) and those at higher risk of detection bias (e.g,. quality of life outcomes). Studies of different designs will be evaluated within the context of their respective designs. Thus, RCTs will be rated as good, fair, or poor, and observational studies will be separately rated as good, fair, or poor.
E. Data Synthesis
We will begin by summarizing key features of the included studies for each KQ. To the degree that data are available, we will abstract information on study design; patient characteristics; clinical settings; diagnostic tools; and intermediate, final, and adverse event outcomes. We will order our findings by treatment or diagnostic comparison and then within these comparisons by outcome with long-term final outcomes emphasized.
We will review and highlight studies using a hierarchy-of-evidence approach. The best evidence available will be the focus of our synthesis for each key question. If high quality evidence is not available we will describe any lower quality evidence we were able to identify, but we will underscore the issues that make it lower quality and the uncertainties in our findings. We will assess and state whether the inclusion of lower quality studies would change any of our conclusions and perform sensitivity analyses excluding this evidence where appropriate.
We will then determine the feasibility of completing a quantitative synthesis (i.e., meta-analysis). Feasibility depends on the volume of relevant literature (we will require 3 appropriate studies to consider meta-analysis of intervention studies and 3 to consider meta-analysis of observational diagnostic test studies), conceptual homogeneity of the studies, and completeness of the reporting of results. For meta-analyses summarizing the sensitivity and sensitivity of diagnostic rests, convergence problems of the bivariate random effects methodology may be encountered and we may need to revert to analysis sensitivity and specificity separately. When a meta-analysis is appropriate, we will use random-effects models to synthesize the available evidence quantitatively. We will test for heterogeneity using graphical displays and test statistics (Q and I2 statistics), while recognizing that the ability of statistical methods to detect heterogeneity may be limited. We will present summary estimates, standard errors, and confidence intervals. We anticipate that intervention effects may be heterogeneous. If there are sufficient studies, we will perform subgroup analyses and/or meta-regression analyses to examine these hypotheses. We will perform quantitative and qualitative syntheses separately by study type and discuss their consistency qualitatively.
Note that we will integrate any newly identified studies with findings from our original report and update any previous meta-analyses with the new studies. To address the anticipated lack of direct comparisons, we will consider multiple treatment comparison analyses or other approaches to indirect comparisons.
Because contextual questions are not reviewed using systematic review methodology, formal data synthesis for this question will not be performed but rather we will summarize the evidence from key informative studies.
F. Grading the Evidence for Each Key Question
At the completion of our review, two reviewers will independently grade the strength of evidence of the outcomes deemed to be of greatest important to decisionmakers and those most commonly reported as key outcomes in the literature including incidence of stroke, major bleeding, and death by adapting an evidence grading scheme recommended by the AHRQ Methods Guide. Conflicts will be resolved through consensus or third-party adjudication. In brief, the approach requires assessment of five domains: study limitations (previously named risk of bias), consistency, directness, precision, and reporting bias, which includes publication bias, outcome reporting, and analysis reporting bias. For intervention trials, these domains affect the confidence in treatment effects. For diagnostic test studies, these factors affect the confidence in estimates of test accuracy and effects on patient management.33 These domains will be considered qualitatively, and a summary rating of high, moderate, or low strength of evidence will be assigned for each outcome after discussion by two reviewers. In some cases, high, moderate, or low ratings will be impossible or imprudent to make, for example, when no evidence is available or when evidence on the outcome is too weak, sparse, or inconsistent to permit any conclusion to be drawn. In these situations, a grade of "insufficient" will be assigned. This four-level rating scale consists of the following definitions:
- High--We are very confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. We believe that the findings are stable, i.e., another study would not change the conclusions.
- Moderate--We are moderately confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has some deficiencies. We believe that the findings are likely to be stable, but some doubt remains.
- Low--We have limited confidence that the estimate of effect lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). We believe that additional evidence is needed before concluding either that the findings are stable or that the estimate of effect is close to the true effect.
- Insufficient--We have no evidence, we are unable to estimate an effect, or we have no confidence in the estimate of effect for this outcome. No evidence is available or the body of evidence has unacceptable deficiencies, precluding reaching a conclusion.
G. Assessing Applicability
We will assess applicability across our key questions using the method described in AHRQ's Methods Guide.24 In brief, this method uses the PICOTS format as a way to organize information relevant to applicability. The most important issue with respect to applicability is whether the outcomes are different across studies that recruit different populations (e.g., age groups, exclusions for comorbidities) or use different methods to implement the interventions of interest; that is, important characteristics are those that affect baseline (control group) rates of events, intervention group rates of events, or both. Example factors include narrow eligibility criteria and exclusion of those with comorbidities, event rates much higher or lower than observed in population-based studies, monitoring practices or visit frequency not used in typical practice, use of substandard alternative therapy, short-term or surrogate outcomes, and standards of care differ markedly from setting of interest. We will use a checklist to guide the assessment of applicability. We will use these data to evaluate the applicability to clinical practice, paying special attention to study eligibility criteria, demographic features of the enrolled population in comparison to the target population, characteristics of the intervention used in comparison with care models currently in use, and clinical relevance and timing of the outcome measures. We will summarize issues of applicability qualitatively.
V. References
- Lopes RD, Crowley MJ, Shah BR, et al. AHRQ Comparative Effectiveness Reviews. Stroke Prevention in Atrial Fibrillation. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15;114(7):e257-354. doi: 10.1161/circulationaha.106.177292. PMID: 16908781.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 9;285(18):2370-5. PMID: 11343485.
- Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994 Aug 1;74(3):236-41. PMID: 8037127.
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46-e215. doi: 10.1161/circulationaha.109.192667. PMID: 20019324.
- Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11(2):281-98. doi: 10.3111/13696990802063425. PMID: 19450086.
- Thrall G, Lane D, Carroll D, et al. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006 May;119(5):448 e1-19. doi: 10.1016/j.amjmed.2005.10.057. PMID: 16651058.
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002 Oct 1;113(5):359-64. PMID: 12401529.
- Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology. 2003 Mar-Apr;22(2):118-23. doi: 10.1159/000068743. PMID: 12629277.
- Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996 Oct;27(10):1760-4. PMID: 8841325.
- Paciaroni M, Agnelli G, Caso V, et al. Atrial fibrillation in patients with first-ever stroke: frequency, antithrombotic treatment before the event and effect on clinical outcome. J Thromb Haemost. 2005 Jun;3(6):1218-23. doi: 10.1111/j.1538-7836.2005.01344.x. PMID: 15892862.
- Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care. 2004 Dec;10(14 Suppl):S451-58; discussion S8-61. PMID: 15696909.
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001 Jun 13;285(22):2864-70. PMID: 11401607.
- Inoue H, Nozawa T, Hirai T, et al. Accumulation of risk factors increases risk of thromboembolic events in patients with nonvalvular atrial fibrillation. Circ J. 2006 Jun;70(6):651-6. PMID: 16723782.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263-72. doi: 10.1378/chest.09-1584. PMID: 19762550.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 02;130(23):e199-267. doi: 10.1161/CIR.0000000000000041. PMID: 24682347.
- Piccini JP, Hernandez AF, Zhao X, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. 2009 Sep 29;54(14):1280-9. doi: 10.1016/j.jacc.2009.04.091. PMID: 19778670.
- Hsu JC, Chan PS, Tang F, et al. Oral Anticoagulant Prescription in Patients With Atrial Fibrillation and a Low Risk of Thromboembolism: Insights From the NCDR PINNACLE Registry. JAMA Intern Med. 2015 Jun;175(6):1062-5. doi: 10.1001/jamainternmed.2015.0920. PMID: 25867280.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016 Oct 07;37(38):2893-962. doi: 10.1093/eurheartj/ehw210. PMID: 27567408.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health. 1998 Jun;52(6):377-84. PMID: 9764259.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health Care Program. Journal of Clinical Epidemiology. 2010 May;63(5):513-23. PMID: 19595577.
- Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine. 2011;155(8):529-36. PMID: 22007046.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013 Nov 28;369(22):2093-104. doi: 10.1056/NEJMoa1310907. PMID: 24251359.
- Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality. Available at: https://effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview/. Accessed May 7, 2012.
- AHRQ Methods for Effective Health Care. In: Chang SM, Matchar DB, Smetana GW, Umscheid CA, eds. Methods Guide for Medical Test Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012.
- Dechartres A, Altman DG, Trinquart L, et al. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. Jama. 2014 Aug 13;312(6):623-30. doi: 10.1001/jama.2014.8166. PMID: 25117131.
- Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. Bmj. 2013 Apr 24;346:f2304. doi: 10.1136/bmj.f2304. PMID: 23616031.
- Lopes RD, Crowley MJ, Shah BR, et al. Stroke Prevention in Atrial Fibrillation. Rockville (MD); 2013.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011 Oct 18;343:d5928. doi: 10.1136/bmj.d5928. PMID: 22008217.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org. The Cochrane Collaboration; 2011.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016 Oct 12;355:i4919. doi: 10.1136/bmj.i4919. PMID: 27733354.
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed June 5, 2017.
- Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10. doi: https://dx.doi.org/10.1136/bmj.39500.677199.AE. PMID: 18483053.
VI. Abbreviations
- ABC = age, biomarkers, clinical history
- AF = atrial fibrillation
- AHRQ = Agency for Healthcare Research and Quality
- ATRIA = age, female, diabetes, congestive heart failure, hypertension, proteinuria, eGFR <45 or ESRD
- CDSR = Cochrane Database of Systematic Reviews
- CHADS2 = congestive heart failure, hypertension, age >75, diabetes, stroke/TIA
- CHADS2-VASc = congestive heart failure/left ventricular ejection fraction ≤ 40%, hypertension, age ≥ 75, diabetes, stroke/TIA/thromboembolism, vascular disease, age 65-74, female
- CKD = chronic kidney disease
- CT = computed tomography
- DM = diabetes mellitus
- DTI = direct thrombin inhibitor
- DVT = deep vein thrombosis
- eGFR = estimate glomerular filtration rate
- EPC = Evidence-based Practice Center
- ESRD = end-stage renal disease
- HAS-BLED = hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (>65), drugs/alcohol concomitantly
- HEMORR2HAGES = hepatic or renal disease, ethanol (alcohol) abuse, malignancy, older (>75), reduced platelet count or function, rebleeding risk, hypertension (uncontrolled), anemia, genetic factors, excessive fall risk, stroke history
- ICH = intracerebral hemorrhage
- INR = international normalized ratio
- IV = intravenous
- KQ = key question
- LAA = left atrial appendage
- MRI = magnetic resonance imaging
- PE = pulmonary embolism
- PICOTS = Populations, Interventions, Comparators, Outcomes, Timing, Settings
- QUADAS = quality assessment of diagnostic accuracy studies
- RCT = randomized controlled trial
- SRDR = Systematic Review Data Repository
- TIA = transient ischemic attack
- TEE = transthoracic echo
- TTE = transesophageal echo
- VKA = vitamin K antagonist
VII. Summary of Protocol Amendments
| Date | Section | Original Protocol | Revised Protocol | Rationale |
|---|---|---|---|---|
| 6/28/2017 | IV. Methods, Table 2. PICOTS and Inclusion and exclusion criteria | Bleeding outcomes of interest were specified in the original protocol as below:
|
Bleeding outcomes of interest were revised to the following:
|
The categorization of bleeding outcomes of interest was revised to increase clarity and specificity in the terminology used, and to align with the evaluation of bleeding outcomes in the systematic review from 2013. |
| 6/28/2017 | III. Analytic Framework | The framework in the original protocol presented bleeding outcomes as:
|
The revised framework reflects the clarified list of bleeding outcomes:
|
This modification aligns the framework diagram with the revised bleeding outcome terminology described above for Table 2. |
| 6/28/2017 | Appendix A: PubMed Search Strategies | The search strategies presented in Appendix A of the original protocol were designed separately for each KQ using outcome-specific search terms. | The revised search strategies are designed to use risk and safety term concepts. For transparency and reproducibility, the revised strategies have been added to Appendix A and presented in their entirety. |
These revisions improve the sensitivity and specificity of the PubMed searches. Internal peer review of the literature searches presented in the original protocol led to an improved approach that used the PubMed IDs of the articles included in the 2013 systematic review report to guide the searches for this update. This approach enabled us to replace the tools and individual risk factors in KQ1 and KQ2 with a single risk concept, resulting in increased sensitivity and specificity. If set to the date limit of the 2013 review searches, this revised search strategy captures all of the KQ1 and KQ2 includes from the 2013 report. In the KQ3 search, we have incorporated a similar concept for risk/safety. Again, if set to the date limits of the 2013 review, this revised PubMed search strategy captures all KQ3 studies from the 2013 review other than one article that is not indexed in PubMed. |
| 10/26/2017 | IV. Methods, Table 2. PICOTS and Inclusion and exclusion criteria - Setting | None |
|
This revision excludes areas of the world where clinical practice significantly differs from standards in the United States. |
| 10/26/2017 | IV. Methods, Table 2. PICOTS and Inclusion and exclusion criteria – Study Design | Study design exclusions originally specified the following:
|
Study design exclusions were revised to the following:
|
Observational studies with fewer than 1000 patients targeting only pharmacological interventions were determined to be insufficiently powered to modify decision making beyond evidence available in the remaining literature to be searched. Note this exclusion does not restrict observational studies which target nonpharmacological interventions where evidence is more sparse and smaller studies may impact our findings. |
VIII. Key Informants/Technical Experts and Review of Key Questions
Key Informants are the end users of research, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making health care decisions.
Technical Experts constitute a multidisciplinary group of clinical, content, and methodological experts who provide input in defining populations, interventions, comparisons, and outcomes and identify particular studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development.
Key Informants and Technical Experts were included in two multi-stakeholder virtual workshops by PCORI in December 2016 and January 2017. The workshops reviewed scoping for the updated review, prioritization of key questions, a discussion of where the evidence base has accumulated since the prior review and emerging issues in AF. This stroke prevention in AF protocol was developed based upon findings from the January 2017 multi-stakeholder virtual workshop. Key Informants and Technical Experts do not do analysis of any kind nor do they contribute to the writing of the report. They have not reviewed the report, except as given the opportunity to do so through the peer or public review mechanism.
IX. Peer Reviewers
Peer Reviewers, representing the diversity of perspectives included in the definition of "Key Informants" and "Technical Experts" above, are invited to provide written comments on the draft report based on their clinical, content, or methodological expertise. The EPC considers all peer review comments on the draft report in preparation of the final report. Peer Reviewers do not participate in writing or editing of the final report or other products. The final report does not necessarily represent the views of individual reviewers. The EPC will complete a disposition of all peer review comments. The disposition of comments for systematic reviews and technical briefs will be published 3 months after the publication of the evidence report.
Potential Peer Reviewers must disclose any financial conflicts of interest greater than $10,000 and any other relevant business or professional conflicts of interest. Invited Peer Reviewers may not have any financial conflict of interest greater than $10,000. Peer Reviewers who disclose potential business or professional conflicts of interest may submit comments on draft reports through the public comment mechanism.
XII. EPC Team Disclosures
No team members have financial conflicts of interest.
XIII. Role of the Funder
This project was funded under Contract No. HHSA290201500004I from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services, through funds provided by a partnership with the Patient-Centered Outcomes Research Institute (PCORI). The AHRQ Task Order Officer reviewed contract deliverables for adherence to contract requirements and quality. The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by PCORI, the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
XIV. Registration
This protocol will be registered in the international prospective register of systematic reviews (PROSPERO).
Appendix A: PubMed Search Strategies
| Search | Query |
|---|---|
| #1 | "Atrial Fibrillation"[Mesh] OR "Atrial Flutter"[Mesh] OR "atrial fibrillation"[tiab] OR afib[tiab] OR "atrial flutter"[tiab] |
| #2 | "Magnetic Resonance Imaging"[Mesh] OR "Cardiac Imaging Techniques"[Mesh] OR "Tomography, X-Ray Computed"[Mesh] OR "International Normalized Ratio"[Mesh] OR "Time Factors"[Mesh] OR CHADS2[tiab] OR "CHADS2-VASc"[tiab] OR CHA2DS2-VASc[tiab] OR “Framingham”[tiab] OR “ABC”[tiab] OR "international normalized ratio"[tiab] OR "international normalized ratios"[tiab] OR INR[tiab] OR duration[tiab] OR frequen*[tiab] OR echocardiogr*[tiab] OR echo[tiab] OR echoes[tiab] OR echos[tiab] OR TTE[tiab] OR TEE[tiab] OR "CT-scan"[tiab] OR "CT-scans"[tiab] OR "Computed Tomography"[tiab] OR "CAT scan"[tiab] OR "CAT scans"[tiab] OR "Computer Assisted Tomography"[tiab] OR "Computerized Axial Tomography"[tiab] OR "Magnetic Resonance Imaging"[tiab] OR MRI[tiab] OR MRIs[tiab] |
| #3 | "Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "brain ischemia"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR ((brain[tiab] OR cerebral[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR “cerebrovascular accident”[tiab] OR “cerebrovascular accidents”[tiab] OR CVA[tiab] OR CVAs[tiab] OR “brain vascular accident”[tiab] OR “brain vascular accidents”[tiab] |
| #4 | #1 AND #2 AND #3 |
| #5 | ("diagnosis"[Mesh] OR "diagnosis"[Subheading] OR "diagnosis"[tiab] OR diagnose[tiab] OR diagnoses[tiab] OR diagnostic[tiab] OR diagnosed[tiab] OR "treatment outcome"[Mesh] OR "Sensitivity and Specificity"[Mesh] OR "decision making"[Mesh] OR outcome[tiab] OR outcomes[tiab] OR reliability[tiab] OR accuracy[tiab] OR accurate[tiab] OR sensitive[tiab] OR Sensitivity[tiab] OR specificity[tiab] OR valid[tiab] OR validity[tiab] OR validation[tiab] OR decision[tiab] OR decisions[tiab] OR assessment[tiab]) NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #6 | #5 AND #4 |
| #7 | #6 NOT (animals[mh] NOT humans[mh]) AND English[lang] AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
| Search | Query |
|---|---|
| #1 | "Atrial Fibrillation"[Mesh] OR "Atrial Flutter"[Mesh] OR "atrial fibrillation"[tiab] OR afib[tiab] OR "atrial flutter"[tiab] |
| #2 | "Age Factors"[Mesh] OR "Dementia"[Mesh] OR "Cognitive Dysfunction"[Mesh] OR "Accidental Falls"[Mesh] OR "International Normalized Ratio"[Mesh] OR "Heart Diseases"[Mesh] OR "Time Factors"[Mesh] OR "Kidney Failure, Chronic"[Mesh] OR "Diabetes Mellitus"[Mesh] OR "Sex Factors"[Mesh] OR "Population Groups"[Mesh] OR "Neoplasms"[Mesh] OR "HIV"[Mesh] OR "HIV Long-Term Survivors"[Mesh] OR "HIV-Associated Lipodystrophy Syndrome"[Mesh] OR "HIV Infections"[Mesh] OR age[tiab] OR dementia[tiab] OR fall[tiab] OR falls[tiab] OR INR[tiab] OR "international normalized ratio"[tiab] OR "international normalized ratios"[tiab] OR paroxysmal[tiab] OR persistent[tiab] OR permanent[tiab] OR stratification[tiab] OR classification[tiab] OR schema[tiab] OR "cognitive impairment"[tiab] OR "cognitive impairments"[tiab] OR "cognitive dysfunction"[tiab] OR "cognitive dysfunctions"[tiab] OR "cognitive decline"[tiab] OR "cognitive declines"[tiab] OR ((prior[tiab] OR previous[tiab] OR first[tiab]) AND stroke[tiab]) OR has-bled[tiab] OR HEMORR2HAGES[tiab] OR "Anticoagulation and Risk Factors in Atrial Fibrillation"[tiab] OR ATRIA[tiab] OR “Bleeding Risk”[tiab] OR duration[tiab] OR frequen*[tiab] OR “heart disease”[tiab] OR "heart diseases"[tiab] OR "cardiac disease"[tiab] OR "cardiac diseases"[tiab] OR "chronic kidney failure"[tiab] OR "chronic renal failure"[tiab] OR "end stage renal disease"[tiab] OR ESRD[tiab] OR “end stage kidney disease”[tiab] OR ESKD[tiab] OR “renal function”[tiab] OR diabetes[tiab] OR diabetic[tiab] OR sex[tiab] OR race[tiab] or ethnic*[tiab] OR cancer[tiab] OR cancers[tiab] OR tumor[tiab] OR tumors[tiab] OR tumours[tiab] OR tumour[tiab] OR carcinoma[tiab] OR carcinomas[tiab] OR neoplasm[tiab] or neoplasms[tiab] OR HIV[tiab] OR "human immunodeficiency virus"[tiab] OR "HIV-infected"[tiab] |
| #3 | "Intracranial Hemorrhages"[Mesh] OR "Hemorrhage"[Mesh:noexp] OR hemorrhage[tiab] OR hemorrhages[tiab] OR hemorrhaging[tiab] OR bleeding[tiab] OR bleed[tiab] OR bleeds[tiab] OR hemorrhagic[tiab] OR haemorrhage[tiab] OR haemorrhages[tiab] OR haemorrhaging[tiab] OR haemorrhagic[tiab] |
| #4 | #1 AND #2 AND #3 |
| #5 | ("diagnosis"[Mesh] OR "diagnosis"[Subheading] OR "diagnosis"[tiab] OR diagnose[tiab] OR diagnoses[tiab] OR diagnostic[tiab] OR diagnosed[tiab] OR "treatment outcome"[Mesh] OR "Sensitivity and Specificity"[Mesh] OR "decision making"[Mesh] OR outcome[tiab] OR outcomes[tiab] OR reliability[tiab] OR accuracy[tiab] OR accurate[tiab] OR sensitive[tiab] OR Sensitivity[tiab] OR specificity[tiab] OR valid[tiab] OR validity[tiab] OR validation[tiab] OR decision[tiab] OR decisions[tiab] OR assessment[tiab]) NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #6 | #5 AND #4 |
| #7 | #6 NOT (animals[mh] NOT humans[mh]) AND English[lang] AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
| Search | Query |
|---|---|
| #1 | "Atrial Fibrillation"[Mesh] OR "atrial fibrillation"[tiab] OR afib[tiab] OR "atrial flutter"[Mesh] OR "atrial flutter"[tiab] |
| #2 | "Anticoagulants"[Mesh] OR "Warfarin"[Mesh] OR "Heparin"[Mesh] OR "Vitamin K/antagonists and inhibitors"[Mesh] OR "Rivaroxaban"[Mesh] OR Antithrombins[Pharmacological Action] OR "Dabigatran"[Mesh] OR "Blood Coagulation Factor Inhibitors"[Mesh] OR "Anticoagulants"[Pharmacological Action] OR "Factor Xa Inhibitors"[Pharmacological Action] OR "apixaban"[Supplementary Concept] OR "edoxaban"[Supplementary Concept] OR warfarin[tiab] OR coumadin[tiab] OR "vitamin k"[tiab] OR enoxaparin[tiab] OR lovenox[tiab] OR rivaroxaban[tiab] OR xarelto[tiab] OR dabigatran[tiab] OR pradaxa[tiab] OR heparin[tiab] OR apixaban[tiab] OR eliquis[tiab] OR edoxaban[tiab] OR lixiana[tiab] OR anticoagulant[tiab] OR anticoagulants[tiab] OR anticoagulation[tiab] OR "thrombin inhibitor"[tiab] OR "thrombin inhibitors"[tiab] OR antithrombin[tiab] OR antithrombins[tiab] OR antithrombotic[tiab] OR "factor Xa inhibitor"[tiab] OR "factor Xa inhibitors"[tiab] OR "Blood clotting inhibitor"[tiab] OR "blood clotting inhibitors"[tiab] |
| #3 | "Platelet Aggregation Inhibitors"[Mesh] OR "Aspirin"[Mesh] OR "Dipyridamole"[Mesh] OR "Platelet Aggregation Inhibitors"[Pharmacological Action] OR clopidogrel[Supplementary Concept] OR clopidogrel[tiab] OR plavix[tiab] OR aspirin[tiab] OR dipyridamole[tiab] OR aggrenox[tiab] OR persantine[tiab] OR curantil[tiab] OR antiplatelet[tiab] OR anti-platelet[tiab] OR antiplatelets[tiab] OR anti-platelets[tiab] OR "platelet aggregation inhibitors"[tiab] OR "platelet aggregation inhibitor"[tiab] OR "platelet inhibitors"[tiab] OR "platelet inhibitor"[tiab] OR "platelet antagonists"[tiab] OR "platelet antagonist"[tiab] |
| #4 | Atrial Appendage/surgery[mesh] OR "Septal Occluder Device"[Mesh] OR “atrial appendage”[tiab] OR "atrial appendages"[tiab] OR "atrium appendage"[tiab] OR "atrium appendages"[tiab] OR "auricular appendage"[tiab] OR "auricular appendages"[tiab] OR LAA[tiab] OR occluder[tiab] OR occluders[tiab] OR occlusion[tiab] OR AMPLATZER[tiab] OR AtriClip[tiab] OR PLAATO[tiab] OR Watchman[tiab] OR (atrial[tiab] AND modification[tiab]) OR lariat[tiab] OR atricure[tiab] |
| #5 | "Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "brain ischemia"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR ((brain[tiab] OR cerebral[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR “cerebrovascular accident”[tiab] OR “cerebrovascular accidents”[tiab] OR CVA[tiab] OR CVAs[tiab] OR “brain vascular accident”[tiab] OR “brain vascular accidents”[tiab] |
| #6 | #1 AND (#2 OR #3 OR #4) AND #5 |
| #7 | "evaluation studies"[Publication Type] OR "evaluation studies as topic"[Mesh] OR "evaluation study"[tiab] OR evaluation studies[tiab] OR "intervention study"[tiab] OR "intervention studies"[tiab] OR "case-control studies"[Mesh] OR "case-control"[tiab] OR "cohort studies"[Mesh] OR cohort[tiab] OR "longitudinal studies"[Mesh] OR "longitudinal"[tiab] OR longitudinally[tiab] OR "prospective"[tiab] OR prospectively[tiab] OR "retrospective studies"[Mesh] OR "retrospective"[tiab] OR "follow up"[tiab] OR "comparative study"[Publication Type] OR "comparative study"[tiab] OR systematic[subset] OR "meta-analysis"[Publication Type] OR "meta-analysis as topic"[Mesh] OR "meta-analysis"[tiab] OR "meta-analyses"[tiab] OR randomized controlled trial[Publication Type] OR controlled clinical trial[Publication Type] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR placebo[tiab] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR Clinical trial[Publication Type] OR "clinical trial"[tiab] OR "clinical trials"[tiab] NOT (Editorial[ptyp] OR Letter[ptyp] OR Case Reports[ptyp] OR Comment[ptyp]) |
| #8 | #7 AND #6 |
| #9 | #8 NOT (animals[mh] NOT humans[mh]) AND English[lang] AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication]) |
Contextual Question Search
((("Atrial Fibrillation"[Mesh] OR "Atrial Flutter"[Mesh] OR "atrial fibrillation"[tiab] OR afib[tiab] OR "atrial flutter"[tiab])) AND ("Stroke"[Mesh] OR "Thromboembolism"[Mesh] OR "brain ischemia"[Mesh] OR stroke[tiab] OR strokes[tiab] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR thromboembolic[tiab] OR ((brain[tiab] OR cerebral[tiab]) AND (ischemia[tiab] OR ischaemia[tiab] OR ischemias[tiab] OR ischaemias[tiab])) OR (transient[tiab] AND (ischemic[tiab] OR ischaemic[tiab] OR ischaemia[tiab] OR ischemia[tiab]) AND (attack[tiab] OR attacks[tiab])) OR TIA[tiab] OR TIAs[tiab] OR "cerebrovascular accident"[tiab] OR "cerebrovascular accidents"[tiab] OR CVA[tiab] OR CVAs[tiab] OR "brain vascular accident"[tiab] OR "brain vascular accidents"[tiab])) AND ("Clinical Decision-Making"[Mesh] OR "Decision Support Systems, Clinical"[Mesh] OR "Decision Making, Computer-Assisted"[Mesh] OR "Decision Support Techniques"[Mesh] OR "Decision Making"[Mesh] OR "Decision Theory"[Mesh] OR "Medical Order Entry Systems"[Mesh] OR "Point-of-Care Systems"[Mesh] OR "decision"[tiab]) AND ("2011/08/01"[Date - Publication] : "3000"[Date - Publication])